Arteriovenous Fistula Devices Market 2029: Surprising Facts and Future Projections Exposed
Strong 8k brings an ultra-HD IPTV experience to your living room and your pocket.
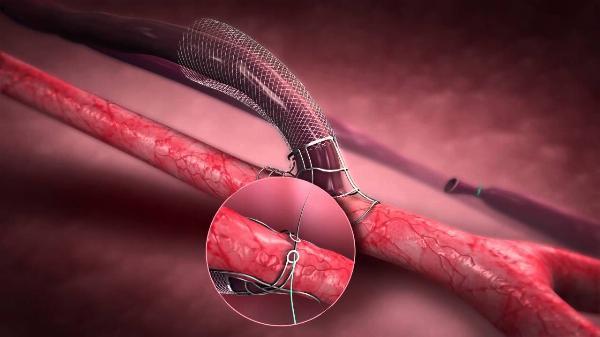
According to the TechSci Research report titled “Arteriovenous Fistula Devices Market – Global Industry Size, Share, Trends, Competition Forecast & Opportunities, 2019-2029F”, the Global Arteriovenous Fistula (AVF) Devices Market was valued at USD 300.12 million in 2023 and is expected to grow at a robust CAGR of 7.25% through 2029. The market's significant expansion is driven by strategic collaborations, technological advancements, and supportive government initiatives aimed at enhancing healthcare infrastructure and accessibility to quality treatment options.
Industry Key Highlights
- Market Size and Growth: The market reached USD 300.12 million in 2023 and is forecasted to grow at a CAGR of 7.25% by 2029.
- Key Drivers: Rising prevalence of chronic kidney disease (CKD) and end-stage renal disease (ESRD), technological advancements in AVF devices, and supportive healthcare initiatives.
- Dominant Segments: Hospitals are the leading end-users due to their advanced infrastructure and availability of skilled healthcare professionals.
- Top Companies: Key players include Medtronic Plc, Becton, Dickinson and Company, Teleflex Incorporated, Fresenius Medical Care, and B. Braun SE.
- Geographical Reach: The market has a global presence, with significant growth observed in North America, Europe, and the Asia-Pacific regions.
Browse market data Figures spread through 280 Pages and an in-depth TOC on "Global Arteriovenous Fistula Devices Market” - https://www.techsciresearch.com/report/arteriovenous-fistula-devices-market/23877.html
What Are the Emerging Trends in the Arteriovenous Fistula Devices Market?
The global AVF devices market is evolving rapidly, driven by several emerging trends:
- Technological Innovations: Ongoing advancements in AVF device technology, such as the development of bioengineered fistulas and minimally invasive devices, are enhancing the safety and efficacy of AVF procedures.
- Shift Towards Outpatient and Ambulatory Settings: As healthcare systems emphasize reducing hospital stays, there is a growing trend towards performing AVF procedures in outpatient and ambulatory care settings.
- Patient-Centric Solutions: Manufacturers are focusing on developing patient-friendly devices that reduce discomfort and improve the overall dialysis experience.
- Increased Focus on Education and Training: Efforts to educate healthcare providers on AVF creation and management are expanding, leading to better patient outcomes and reduced complication rates.
What Are the Key Drivers of the Market?
Several factors are driving the growth of the arteriovenous fistula devices market:
- Rising Prevalence of CKD and ESRD: The increasing incidence of CKD and ESRD worldwide has led to a higher demand for hemodialysis, where AVFs are essential for vascular access.
- Government Initiatives: Supportive government policies aimed at enhancing healthcare infrastructure and providing access to quality care are boosting the adoption of AVF devices. These initiatives include funding for dialysis programs and incentives for healthcare providers.
- Technological Advancements: Innovations in AVF devices, such as improved materials, designs, and functionalities, are enhancing patient outcomes and reducing the risk of complications.
- Growing Awareness and Acceptance: Healthcare campaigns promoting the benefits of AVFs over alternative access methods, such as catheters, are increasing the adoption of these devices among patients and healthcare providers.
- Aging Population: The global aging population is contributing to the rising prevalence of kidney-related diseases, thereby driving the demand for AVF devices.
How Does the Regulatory Scenario Shape this Industry?
The regulatory landscape significantly impacts the AVF devices market, guiding product development, approval, and market entry. Key regulatory considerations include:
- Compliance with International Standards: AVF devices must adhere to stringent international standards, such as ISO 13485 for medical devices, ensuring high quality and safety.
- FDA and CE Approvals: In the U.S., AVF devices require approval from the Food and Drug Administration (FDA), while in Europe, the CE marking is necessary for market access. These regulatory bodies ensure that devices meet rigorous safety and efficacy criteria before reaching the market.
- Incentives for Innovation: Some governments offer incentives and fast-track approval processes for innovative medical devices that address unmet clinical needs, encouraging further advancements in AVF technology.
- Focus on Patient Safety and Data Protection: Regulatory guidelines emphasize patient safety, with strict requirements for device performance, biocompatibility, and data protection, particularly when devices are used in conjunction with digital health platforms.
What Are the Top Segments in the Arteriovenous Fistula Devices Market?
The market is segmented by type, end-use, regional distribution, and company. Key segments include:
- By Type: The market offers a range of AVF devices, including grafts, needles, and catheters, with grafts being widely preferred due to their durability and effectiveness in maintaining vascular access.
- By End-Use: Hospitals dominate the end-use segment due to their comprehensive care capabilities, including advanced surgical infrastructure and access to specialized healthcare professionals. Other significant end-users include ambulatory surgical centers and dialysis clinics.
- By Region: North America holds the largest market share, attributed to the region's advanced healthcare infrastructure and high prevalence of CKD and ESRD. The Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare investments and rising disease awareness.
Competitive Analysis
The global AVF devices market is characterized by intense competition, with major players focusing on strategic partnerships, product innovation, and geographic expansion to maintain their market position. Leading companies include:
- Medtronic Plc: A global leader in medical technology, Medtronic offers a broad portfolio of AVF devices designed to enhance patient outcomes and streamline hemodialysis procedures.
- Becton, Dickinson and Company: BD provides a wide range of vascular access devices, leveraging its extensive experience in medical devices to improve the safety and efficiency of AVF creation and maintenance.
- Teleflex Incorporated: Known for its innovative vascular access solutions, Teleflex is a key player in the AVF devices market, focusing on enhancing patient comfort and reducing complication rates.
- Fresenius Medical Care AG & Co. KGaA: As a leading provider of dialysis products and services, Fresenius offers comprehensive AVF solutions that integrate seamlessly into its dialysis care continuum.
- B. Braun SE: B. Braun’s expertise in medical devices extends to AVF solutions, where it emphasizes safety, durability, and ease of use to improve patient care.
Download Free Sample Report - https://www.techsciresearch.com/sample-report.aspx?cid=23877
Customers can also request for 10% free customization on this report
Future Outlook: What Lies Ahead for the Market?
The AVF devices market is poised for significant growth, driven by technological advancements, increasing global awareness of CKD and ESRD, and supportive regulatory environments. Future market growth is expected to be shaped by:
- Innovation in Device Technology: Ongoing R&D efforts are likely to yield next-generation AVF devices with improved features, such as enhanced biocompatibility, longer lifespan, and easier implantation procedures.
- Expansion in Emerging Markets: Rapidly developing healthcare infrastructure in emerging economies like China, India, and Brazil presents significant growth opportunities for AVF device manufacturers.
- Rising Demand for Personalized Care: As the healthcare industry moves towards personalized treatment approaches, there will be a growing need for AVF devices tailored to individual patient needs and anatomical considerations.
- Collaborations and Partnerships: Increased collaboration between healthcare providers, research institutions, and device manufacturers will drive innovation and expand access to advanced AVF solutions.
Benefits of the Research Report
The research report offers comprehensive insights into the AVF devices market, including:
- Market Analysis: Detailed analysis of market size, growth rates, and key drivers influencing the market.
- Competitive Landscape: In-depth evaluation of major players, their strategies, and market positioning.
- Emerging Trends: Identification of key trends and potential opportunities in the AVF devices market.
- Regulatory Insights: Analysis of the regulatory framework and its impact on market dynamics.
- Future Projections: Forecasts of market growth and emerging opportunities through 2029.
- Segment Analysis: Detailed examination of top segments, including type, end-use, and regional distribution.
This report serves as an invaluable resource for stakeholders, including investors, healthcare providers, and industry professionals, seeking to understand the dynamics of the AVF devices market and capitalize on emerging opportunities.
“The primary driver of the Arteriovenous Fistula Devices Market is the escalating prevalence of chronic kidney diseases globally. With an increasing number of patients requiring hemodialysis for end-stage renal disease, there is a growing demand for arteriovenous fistula devices to establish reliable vascular access. Advancements in medical technology have led to the development of innovative devices, enhancing their efficacy and safety profiles. The supportive government initiatives and robust healthcare infrastructure further fuel market growth by facilitating greater accessibility to medical services and products. Collectively, these factors propel the expansion of the Arteriovenous Fistula Devices Market, catering to the rising needs of patients and healthcare providers worldwide” said Mr. Karan Chechi, Research Director of TechSci Research, a research-based management consulting firm.
“Arteriovenous Fistula Devices Market - Global Industry Size, Share, Trends, Opportunity, and Forecast, Segmented By Type (AVF Creation Devices {Surgical Instruments, Vascular Grafts, Angioplasty Balloons, Others}, AVF Monitoring Devices {Doppler Ultrasound, Pressure Monitoring Systems, Others}, AVF Maintenance Devices {Central Venous Catheters, Stents, Others}), By End-use (Hospitals, Cardiac Centers, Others), By Region, and Competition, 2019-2029F”, has evaluated the future growth potential of Global Arteriovenous Fistula Devices Market and provides statistics & information on market size, structure and future market growth. The report intends to provide cutting-edge market intelligence and help decision makers take sound investment decisions. Besides, the report also identifies and analyzes the emerging trends along with essential drivers, challenges, and opportunities in Global Arteriovenous Fistula Devices Market.
Download Free Sample Report - https://www.techsciresearch.com/sample-report.aspx?cid=23877
Related Reports-
Europe Collagen Supplement Market
Europe Dietary Supplement Market
Japan Interferons Market
Contact
US -
Techsci Research LLC
420 Lexington Avenue, Suite 300,
New York, United States- 10170
Tel: +13322586602
Email: [email protected]
Web: https://www.techsciresearch.com/
Note: IndiBlogHub features both user-submitted and editorial content. We do not verify third-party contributions. Read our Disclaimer and Privacy Policyfor details.



![Pediatric Cancer Biomarkers Market: Industry Size and Growth Trends [2029]](https://indibloghub.com/public/images/courses/673194ba88a567460_1731302586.png)
![Vietnam Medical Devices Market: Unlocking Growth Secrets, Trends and Developments [2029]](https://indibloghub.com/public/images/courses/6683a348518645795_1719903048.png)