AI Blog Generation – Mass Content at Lightning Speed!
AI Blog Generation – Mass Content at Lightning Speed!
Biosimilar Market Report 2025-2033: Industry Trends, Segmentation & Forecast Analysis
Written by Business News » Updated on: January 20th, 2025
IMARC Group, a leading market research company, has recently released a report titled “Biosimilar Market Size, Share, Trends and Forecast by Molecule, Indication, Manufacturing Type, and Region, 2025-2033”. The study provides a detailed analysis of the industry, including the biosimilar market share, growth, size, trends and forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
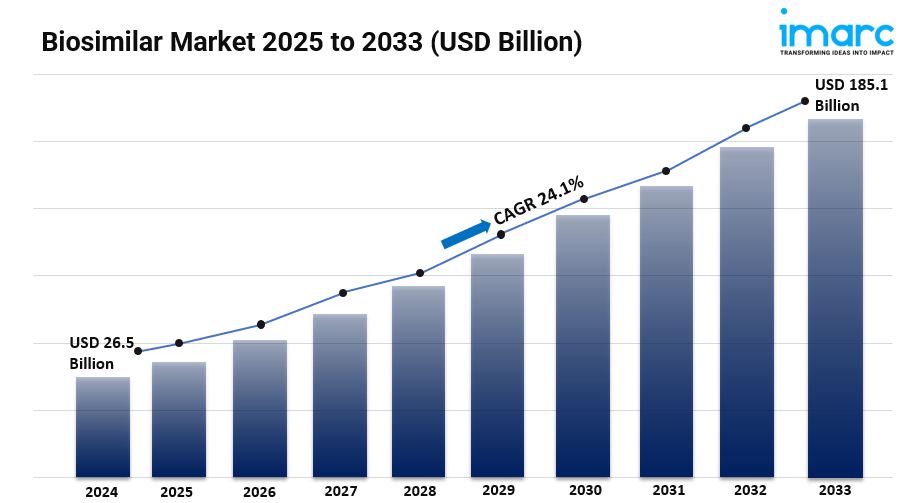
The global biosimilar market size was valued at USD 26.5 Billion in 2024. Looking forward, IMARC Group estimates the market to reach USD 185.1 Billion by 2033, exhibiting a CAGR of 24.1% from 2025-2033.
Request to Get the Sample Report: https://www.imarcgroup.com/biosimilar-market/requestsample
Factors Affecting the Growth of the Biosimilar Industry:
Increasing Healthcare Costs and Demand for Affordable Alternatives
Healthcare costs are rising. This increase drives up the demand for biosimilars. These therapies are crucial for treating complex diseases such as cancer and autoimmune disorders. However, their high prices burden patients and healthcare systems. Biosimilars offer a cost-effective alternative, often 20-30% cheaper than their reference biologics. This affordability is crucial in regions with tight healthcare budgets. High costs restrict access to life-saving treatments. Governments and insurers strive to control these costs. Their goal is to ensure access to essential medications. This drives faster adoption of biosimilars. Awareness of biosimilars' safety and effectiveness among doctors and patients fuels this trend. A competitive market prioritizes affordability without compromising quality.
Regulatory Support and Evolving Approval Pathways
Regulatory agencies around the world now support biosimilars. These products are crucial for giving patients access to important therapies. Biosimilars have clear guidelines. This predictability helps manufacturers. The FDA provides a strong framework. It promotes innovation and ensures patient safety. This supportive environment has led to more biosimilars entering the market. Many have received approval in recent years. As more biosimilars succeed, competition will likely increase. Existing biologic manufacturers may need to adapt their strategies. They might lower prices or invest in new drug development. This shift benefits patients by providing more choices. It also drives research and development in the biopharmaceutical industry. This innovation cycle can result in better therapies.
Rising Chronic Disease Prevalence and Patient Population Growth
Chronic diseases like diabetes, heart disease, and cancer are driving the biosimilar market. As the global population ages, lifestyle-related health issues are on the rise. This increases the demand for effective treatments. Biosimilars offer similar therapeutic benefits as their reference products. This makes them suitable for patients. Manufacturers see big opportunities in emerging markets. These markets have growing populations. Healthcare providers value biosimilars for chronic disease treatment. This leads to more prescriptions. More awareness and education will drive this trend. Consequently, biosimilars will gain a bigger market share. They will be crucial in healthcare.
Speak to An Analyst: https://www.imarcgroup.com/request?type=report&id=497&flag=C
Biosimilar Market Report Segmentation:
Analysis by Molecule:
- Infliximab
- Insulin Glargine
- Epoetin Alfa
- Etanercept
- Filgrastim
- Somatropin
- Rituximab
- Follitropin Alfa
- Adalimumab
- Pegfilgrastim
- Trastuzumab
- Bevacizumab
- Others
Infliximab, driven by biosimilar competition following Remicade patent expiration, leads the 2024 market for autoimmune disease treatments, offering cost-effective options, expanding access, and promoting sustainable healthcare in emerging markets.
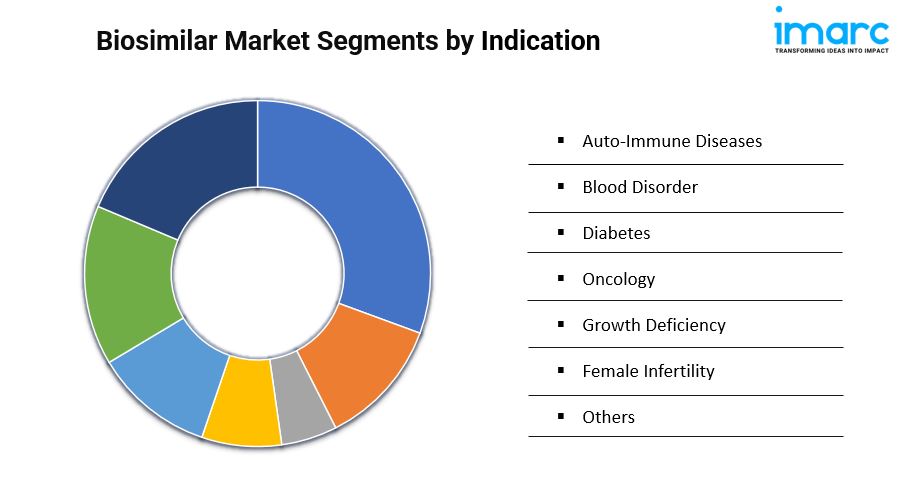
Analysis by Indication:
- Auto-Immune Diseases
- Blood Disorder
- Diabetes
- Oncology
- Growth Deficiency
- Female Infertility
- Others
Biosimilars, driven by affordability and increasing competition, lead the 2024 market in autoimmune diseases, offering cost-effective treatment options for chronic conditions and improving patient access and adherence.
Analysis by Manufacturing Type:
- In-house Manufacturing
- Contract Manufacturing
In-house manufacturing leads the 2024 market, offering biosimilar developers control over quality, cost, and scalability, enabling streamlined processes, reduced reliance on third parties, and fostering innovation for producing high-quality, affordable biologics.
Regional Analysis:
- Europe
- United States
- Japan
- India
- South Korea
- Rest of the World
Europe led the 2024 market due to an early start, a supportive regulatory framework by EMA, increasing awareness and acceptance, and successful launches like Sandoz's Tyruko®, the first natalizumab biosimilar.
Global Biosimilar Market Trends 2025:
By 2025, the biosimilar market will change significantly. Chronic diseases are increasing, creating an urgent need for affordable treatments. This demand is driving biosimilar growth. We expect many more biosimilars to be available by then. New companies will enter the market, while existing ones expand their offerings. Regulatory bodies are likely to speed up approvals, boosting competition. Awareness among doctors and patients about biosimilars' safety and effectiveness will also increase adoption. The move to value-based care is vital. Healthcare systems now prioritize costs and patient outcomes. By 2025, the biosimilar market will see more competition and acceptance. There will be a strong commitment to offering high-quality, affordable therapies for patients.
Top Companies Operated in Biosimilar Industry:
- Sandoz International GmbH
- Pfizer Inc.
- Teva Pharmaceutical Industries Limited
- Celltrion Inc.
- Biocon Limited
- Samsung Biologics
- Amgen, Inc.
- Dr. Reddy's Laboratories Limited
- Stada Arzneimittel Ag.
Key Highlights of the Report:
- Market Performance (2019–2024)
- Market Outlook (2025–2033)
- Market Trends
- Market Drivers and Success Factors
- Impact of COVID-19
- Value Chain Analysis
- Comprehensive mapping of the competitive landscape
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No: (D) +91 120 433 0800
United States: +1–631–791–1145
Disclaimer: We do not promote, endorse, or advertise betting, gambling, casinos, or any related activities. Any engagement in such activities is at your own risk, and we hold no responsibility for any financial or personal losses incurred. Our platform is a publisher only and does not claim ownership of any content, links, or images unless explicitly stated. We do not create, verify, or guarantee the accuracy, legality, or originality of third-party content. Content may be contributed by guest authors or sponsored, and we assume no liability for its authenticity or any consequences arising from its use. If you believe any content or images infringe on your copyright, please contact us at [email protected] for immediate removal.
Copyright © 2019-2025 IndiBlogHub.com. All rights reserved. Hosted on DigitalOcean for fast, reliable performance.