Blog Speed Optimization – Make Google & Users Happy!
Blog Speed Optimization – Make Google & Users Happy!
Deuterated Drugs In The Covid-19 Pandemic
Written by divyaochre » Updated on: June 17th, 2025 127 views

Coronavirus disease 2019 (COVID-19) continues to ravage populations globally. In seeking a potential treatment for COVID-19, deuterated compounds have been (and continue to be) explored as therapeutic agents and internal mass spectrometry standards. We review these in this article, including the drug ‘VV116’, approved as a COVID-19 treatment in December 2021.
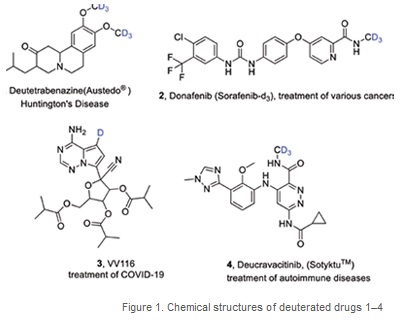
Since the recent (2017) approval by the FDA to commercialise and administer deutetrabenazine (registered name Austedo®) (CAS 1392826-25-3) (1, Figure 1), the first for a deuterated pharmaceutical, there has been a surge of interest in the preparation and testing of deuterated pharmaceutical compounds with improved medicinal properties (safety, efficacy, bioavailability) compared to their non-labelled counterparts. (Figure 1)
This is largely due to C-D bonds requiring slightly more energy to break compared with the C-H bonds. Thus, when situated at the point of metabolism in a drug, the C-D bonds are harder to break (by enzymes, typically cytochrome P450s), therefore slowing down the drug metabolism. This can result in an increase in the drug’s bioavailability and a reduction in the requisite dosage quantity. Altering the bonds at the point of metabolism can also modify the metabolism pathway, resulting in different metabolic products (“metabolic shunting”). Sometimes these alternative metabolites are less toxic than with the original non-labelled drug, making the D-labelled drug safer for use. In most cases, the inclusion of deuterium does not alter the pharmacodynamics of a drug, only its pharmacokinetics. This means that the labelled drug is just as potent as the non-labelled analogue and has the added advantages already described. However, D-labelling can also have a complex effect on intermolecular interactions and binding to enzymes.
The use of D-labelled drugs is also gaining attraction as fluorinated drugs (which offer some of the advantages offered by deuterated drugs) are likely to slowly be phased out because of the persistence and toxicity of trifluoromethyl groups, a component of most metabolites of fluorinated drugs (Hammel 2022).
Since 2017, three other drugs have been approved for prescription and usage [Donafenib/Sorafenib-d3 (2, CAS 1130115-44-4), VV116 (3, CAS 2647442-33-7), and Deucravacitinib/Sotyktu (4, CAS 1609392-27-9), while 19 compounds are under clinical investigation for potential future approval (di Martino 2023). Of the three approved deuterated drugs, the oral remdesivir derivative VV116 was approved in December 2021 for emergency treatment of COVID-19. During the COVID-19 pandemic, many research groups and small industrial companies mobilised resources towards the synthesis and testing of deuterated drugs and biomarkers to find a potential cure for COVID-19 (many companies/groups are still researching in this area). There is a chance that some of these deuterated compounds are still undergoing clinical trials and could, too, meet criteria for approval.
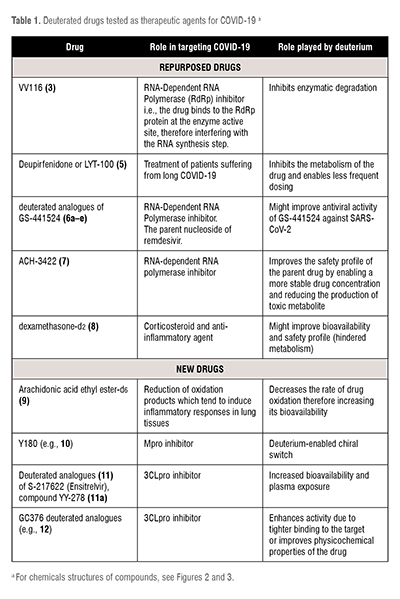
Deuterated drugs explored as treatments for COVID-19 are either repurposed or novel drugs. These have been summarised in the table below. (Table 1)
Deuterated Drugs as Internal Mass Spectrometry (MS) Standards to aid Drug Discovery
Processed internal standards are used to correct errors in detection when using Gas Chromatography (GC) /Liquid Chromatography (LC) coupled with (tandem) Mass Spectrometry (MS) for bioanalysis. This procedure is needed for the development of drugs, for example to understand drug pharmacokinetics and metabolism. The development of drugs for treatment of COVID-19 is no exception. Thus, we list compounds 13–25 as deuterated drugs that have been prepared as internal standards for studies of repurposed drugs for the treatment of COVID-19 in the table below. (Table 2)
In addition to the deuterated drugs or their metabolites used as internal MS standards for studies on the action of their non-deuterated analogues for the treatment of COVID-19, there are also many molecules that have been studied as biomarkers for the detection of COVID-19 or the severity of the disease.
Commercially available deuterated lipids are mostly used for these purposes, as molecules such as lipids, triglycerides and free fatty acids are often indicators (biomarkers) of various (inflammatory) diseases in the body and potential targets for therapeutic agents. Some structures and uses of these compounds can be seen in the review paper by Jansen-van Vuuren et al. 2022.
Explore more: https://www.pharmafocusasia.com/research-development/deuterated-drugs-covid-19-pandemic
Note: IndiBlogHub features both user-submitted and editorial content. We do not verify third-party contributions. Read our Disclaimer and Privacy Policyfor details.
Copyright © 2019-2025 IndiBlogHub.com. All rights reserved. Hosted on DigitalOcean for fast, reliable performance.