Methotrexate Manufacturing Plant Project Report 2024: Processes, Costs, and Considerations
Strong 8k brings an ultra-HD IPTV experience to your living room and your pocket.
Introduction
A Methotrexate Manufacturing Plant Project Report is a critical document for entrepreneurs or businesses interested in setting up a production facility for methotrexate. Methotrexate, a chemotherapy drug used to treat various types of cancer, autoimmune diseases, and ectopic pregnancies, is in high demand in the pharmaceutical sector. Its versatility and proven effectiveness in the treatment of conditions such as rheumatoid arthritis, psoriasis, and certain cancers have contributed to its widespread use. This article outlines the essential steps, processes, market demand, and considerations necessary for setting up a methotrexate manufacturing plant.
What is Methotrexate?
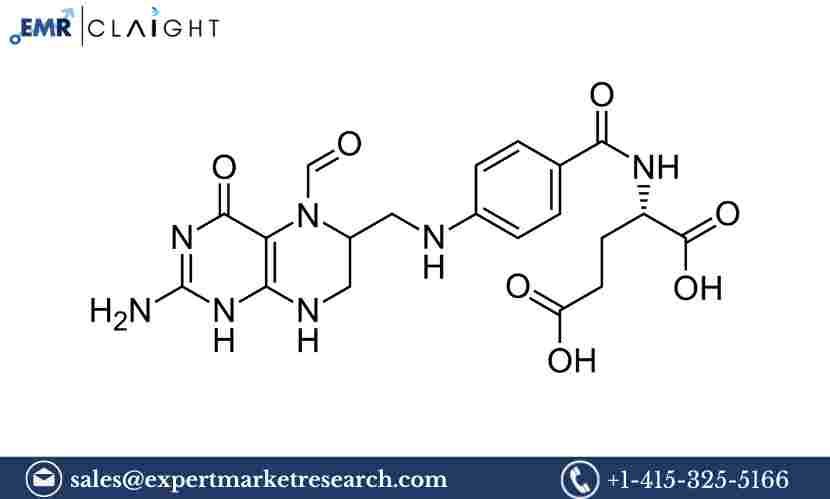
Methotrexate (MTX) is a potent drug that acts by inhibiting the enzyme dihydrofolate reductase, which is involved in the production of DNA and RNA. By interfering with the synthesis of nucleotides, methotrexate prevents cell division, making it highly effective in treating cancers like leukemia, lymphoma, and solid tumors. It is also widely used in the treatment of autoimmune diseases such as rheumatoid arthritis, psoriasis, and Crohn's disease. Methotrexate can be administered orally or via injection, depending on the condition being treated.
Market Demand for Methotrexate
The global market for methotrexate has been expanding due to its extensive medical applications. Some key drivers contributing to the increased demand for methotrexate include:
Increasing Cancer Incidence: The rise in cancer cases globally has led to an increase in demand for chemotherapy drugs, including methotrexate. Its ability to treat various types of cancers, including leukemia, lymphoma, and breast cancer, drives its widespread use in oncology.
Growth in Autoimmune Disease Prevalence: The rising prevalence of autoimmune diseases, such as rheumatoid arthritis and psoriasis, has also contributed to the growing demand for methotrexate. It is one of the most prescribed drugs for these chronic conditions.
Evolving Healthcare Standards: The improvement of healthcare infrastructure and access to medications in emerging markets has led to greater availability and demand for methotrexate in regions such as Asia, Africa, and Latin America.
Generic Methotrexate: The availability of generic versions of methotrexate, following the expiration of patents, has made the drug more affordable and accessible, thus expanding its reach across the global market.
Advancements in Drug Formulations: Innovations in the formulation and delivery methods of methotrexate, such as subcutaneous injections and oral tablets, have also improved patient compliance and contributed to market growth.
Production Process of Methotrexate
The production of methotrexate involves several steps, including chemical synthesis, purification, and formulation. Below is an overview of the general production process:
1. Raw Material Procurement
The raw materials required for the production of methotrexate include various chemical compounds such as pteridine derivatives, aminopterin, and other reactants. These materials are sourced from reliable suppliers who provide high-quality chemicals that meet pharmaceutical-grade standards.
2. Synthesis of Methotrexate
The synthesis of methotrexate is typically carried out in several stages, beginning with the formation of the core structure, followed by the introduction of functional groups to create the final product. The synthesis process involves a series of chemical reactions, including coupling reactions, nucleophilic substitutions, and reductions.
Step 1: Preparation of Pteridine Derivative: The pteridine derivative is synthesized through the reaction of pteridine with other reactants under specific temperature and pressure conditions.
Step 2: Formation of Methotrexate Core Structure: The core structure of methotrexate is formed by coupling various intermediates in the presence of a catalyst. This step creates the essential backbone of the methotrexate molecule.
Step 3: Functional Group Introduction: The final steps involve adding functional groups such as amino and carboxyl groups, which are required for methotrexate’s biological activity.
3. Purification
After the synthesis is completed, methotrexate is subjected to purification to remove any impurities and byproducts that may have been formed during the synthesis process. The purification typically involves techniques such as crystallization, filtration, and chromatography to ensure the final product meets the required purity standards.
4. Formulation and Packaging
Once purified, the methotrexate is formulated into the appropriate dosage forms, such as tablets, injectables, or oral solutions. The formulation process includes mixing the active pharmaceutical ingredient (API) with excipients that aid in the drug's stability, bioavailability, and patient compliance. The formulated drug is then packaged according to regulatory standards, ready for distribution.
Key Equipment for Methotrexate Manufacturing
Setting up a manufacturing plant for methotrexate requires specific equipment for chemical synthesis, purification, and formulation. The key equipment used in the production process includes:
Reactor Vessels: These vessels are used for chemical synthesis, where the raw materials undergo reactions to form methotrexate.
Distillation Columns: Distillation is used to separate and purify methotrexate from other substances based on differences in boiling points.
Crystallizers: These are used for purifying methotrexate by recrystallization, which helps to remove impurities and byproducts.
Filtration Units: Filtration systems are crucial for separating solids from liquids, ensuring that only pure methotrexate is used in the formulation process.
Chromatography Columns: These are used for high-performance liquid chromatography (HPLC) to separate and identify different compounds in the final product.
Tablet Presses: If the methotrexate is to be formulated into tablets, tablet presses are used to compress the active ingredient and excipients into tablet form.
Injection Filling Machines: These are used for packaging methotrexate in injectable form, ensuring precise filling and sealing of vials.
Regulatory Compliance and Safety Considerations
Given that methotrexate is a potent pharmaceutical product, the manufacturing plant must adhere to strict regulatory and safety standards to ensure product quality and safety. Key compliance considerations include:
Good Manufacturing Practices (GMP): GMP guidelines set by regulatory agencies such as the FDA, EMA, and WHO ensure that the manufacturing process for methotrexate meets quality standards and minimizes the risk of contamination. This includes regular quality checks and documentation of production batches.
Environmental Regulations: The production of methotrexate may generate chemical waste and emissions. Manufacturing plants must ensure compliance with local environmental regulations regarding waste disposal, emissions, and water treatment to minimize their environmental impact.
Safety Standards: The production of methotrexate involves the use of hazardous chemicals, so it is essential to follow stringent safety protocols to protect workers. This includes providing proper ventilation, protective equipment, and safety training. Emergency response procedures should also be in place.
Packaging and Labeling Compliance: The packaging of methotrexate must comply with regulatory requirements regarding labeling, dosage instructions, and warnings. Clear, accurate labeling ensures the safe and proper use of the drug.
Financial Considerations and Investment
Establishing a methotrexate manufacturing plant requires significant capital investment. Several factors need to be taken into account when planning the financial aspects of the project:
Capital Investment: The cost of setting up the plant, including the purchase of land, buildings, and equipment, represents a large portion of the capital investment. This also includes the cost of ensuring the facility meets GMP and safety standards.
Operational Costs: Ongoing operational expenses include raw material procurement, labor, utilities, maintenance of equipment, and regulatory compliance costs. The plant should be designed for efficient use of resources to minimize waste and reduce operating costs.
Market Research: Conducting thorough market research to understand the demand for methotrexate and pricing strategies is crucial for projecting potential revenue. Identifying key markets, both domestically and internationally, will help ensure that the plant is economically viable.
Profitability: The profitability of the plant will depend on factors such as production scale, operating efficiency, and the market price of methotrexate. Additionally, strategic partnerships with pharmaceutical companies and healthcare providers can help boost sales.
FAQs
1. What is the primary use of methotrexate?
Methotrexate is primarily used as a chemotherapy drug to treat cancer. It is also used to manage autoimmune diseases such as rheumatoid arthritis and psoriasis.
2. What are the raw materials used in the production of methotrexate?
Key raw materials include pteridine derivatives, aminopterin, and various solvents and reagents needed for the chemical reactions during synthesis.
3. What are the key challenges in methotrexate manufacturing?
Challenges include ensuring the highest level of product purity, adhering to strict regulatory standards, managing chemical waste, and maintaining consistent production quality.
4. How is methotrexate administered?
Methotrexate can be administered orally in tablet form, or through injection, depending on the medical condition being treated.
Media Contact:
Company Name: Claight Corporation
Contact Person: Lewis Fernandas, Corporate Sales Specialist — U.S.A.
Email: [email protected]
Toll Free Number: +1–415–325–5166 | +44–702–402–5790
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Note: IndiBlogHub features both user-submitted and editorial content. We do not verify third-party contributions. Read our Disclaimer and Privacy Policyfor details.


