Osseointegration Implants Market 2029: Untapped Opportunities and Growth Strategies Revealed
Strong 8k brings an ultra-HD IPTV experience to your living room and your pocket.
According to TechSci Research’s report, “Osseointegration Implants Market – Global Industry Size, Share, Trends, Competition, Forecast & Opportunities, 2019-2029F,” the global osseointegration implants market was valued at USD 8.02 billion in 2023. With a steady growth trajectory projected at a compound annual growth rate (CAGR) of 7.34% through 2029, the market is expected to continue expanding. This growth is driven by several key factors, including an aging population, the rising incidence of dental and orthopedic conditions, and continuous advancements in implant technology. This report explores the market dynamics, including the impact of regulatory frameworks, competitive landscape, and future outlook for the global osseointegration implants market.
Browse over XX market data Figures spread through XX Pages and an in-depth TOC on "Global Osseointegration Implants Market” - https://www.techsciresearch.com/report/osseointegration-implants-market/26519.html
How Are Emerging Trends Shaping the Osseointegration Implants Market?
Emerging trends in the osseointegration implants market highlight significant advancements in material science, the shift towards minimally invasive procedures, and the growing role of customized, patient-specific solutions:
Technological Advancements in Implant Materials
Technological innovations have led to the development of advanced implant materials, which are improving both the longevity and effectiveness of implants. Bioactive materials and surface coatings that promote osseointegration are making implants safer and faster to integrate with bone tissue. In addition, 3D printing technologies are enabling the production of patient-specific implants that better fit individual anatomies, thereby enhancing overall outcomes and patient satisfaction.
Preference for Minimally Invasive Procedures
A growing number of patients and healthcare providers are opting for minimally invasive procedures that reduce recovery time, minimize scarring, and lower the risk of complications. Osseointegration implants, often placed using minimally invasive techniques, offer a preferable alternative to traditional, more invasive surgeries. This trend is anticipated to drive increased adoption as patients increasingly seek procedures with shorter recovery times and reduced discomfort.
Personalized and Precision Medicine Approaches
The development of personalized implants designed using digital scanning and 3D modeling to match patient-specific anatomies is gaining traction. Such patient-specific implants are proving to be highly effective in both dental and orthopedic applications, offering improved outcomes and reduced implant rejection rates.
What Are the Key Drivers of Growth in the Osseointegration Implants Market?
The osseointegration implants market is influenced by several growth drivers, including demographic changes, lifestyle factors, and a shift towards value-based healthcare:
Rising Aging Population
Globally, the aging population is growing rapidly, and older adults are more likely to suffer from dental and orthopedic conditions that necessitate the use of implants. Osseointegration implants, with their high durability and compatibility, provide an ideal solution for issues such as tooth loss and joint degeneration that become prevalent with age.
Increasing Prevalence of Chronic Conditions
Lifestyle factors, including sedentary behavior and rising obesity rates, contribute to chronic orthopedic issues that require joint replacement solutions. Osseointegration implants, particularly in knee and hip replacements, address the increasing demand for long-lasting, durable implants in patients with orthopedic degeneration.
Technological Progress in Medical Devices
Continuous advancements in technology, particularly in 3D printing, surface coatings, and bioactive materials, are enhancing the functionality and longevity of implants. These advancements are making osseointegration implants safer, reducing recovery times, and improving the overall success rates of implant procedures.
How Does Regulatory Scenario Shape this Industry?
The regulatory framework surrounding osseointegration implants is critical in determining market growth, as it impacts both the approval process and market acceptance:
Stringent Regulatory Approvals in Europe
In Europe, the regulatory landscape is stringent, with rigorous testing requirements that ensure implant quality and safety. The European Union’s CE marking, which is a hallmark of product compliance with safety and health standards, facilitates confidence among healthcare providers and patients. These regulations not only maintain product quality but also foster innovation among manufacturers seeking to meet high standards.
FDA Regulations in the United States
In the U.S., the Food and Drug Administration (FDA) closely regulates medical implants. Compliance with FDA standards ensures that only the most effective and safe osseointegration implants are approved for use, making this region a vital market for high-quality implants. These standards also promote continuous improvement among manufacturers.
Rising Standards in Asia-Pacific
The Asia-Pacific region is witnessing rising standards for healthcare technology and medical device regulation, with countries like Japan, China, and India investing in healthcare advancements. As the region aligns with international regulatory standards, it presents substantial growth opportunities for osseointegration implants.
Download Free Sample Report - https://www.techsciresearch.com/sample-report.aspx?cid=26519
Top Companies Operating in the Osseointegration Implants Market
Several prominent companies play a crucial role in the osseointegration implants market, driving innovation and offering a range of advanced implant solutions. Key players include:
Medtronic PLC
Stryker Corporation
Smith+Nephew PLC
Zimmer Biomet Holdings, Inc.
Institut Straumann AG
CONMED Corporation
Integrum AB
Dentsply Sirona Inc.
CAMLOG Biotechnologies GmbH
Osstem Implant Co., Ltd.
Top Segments in the Osseointegration Implants Market
The global osseointegration implants market is segmented based on product type, material, end-user, and geographical region. Key segments include:
Product Type
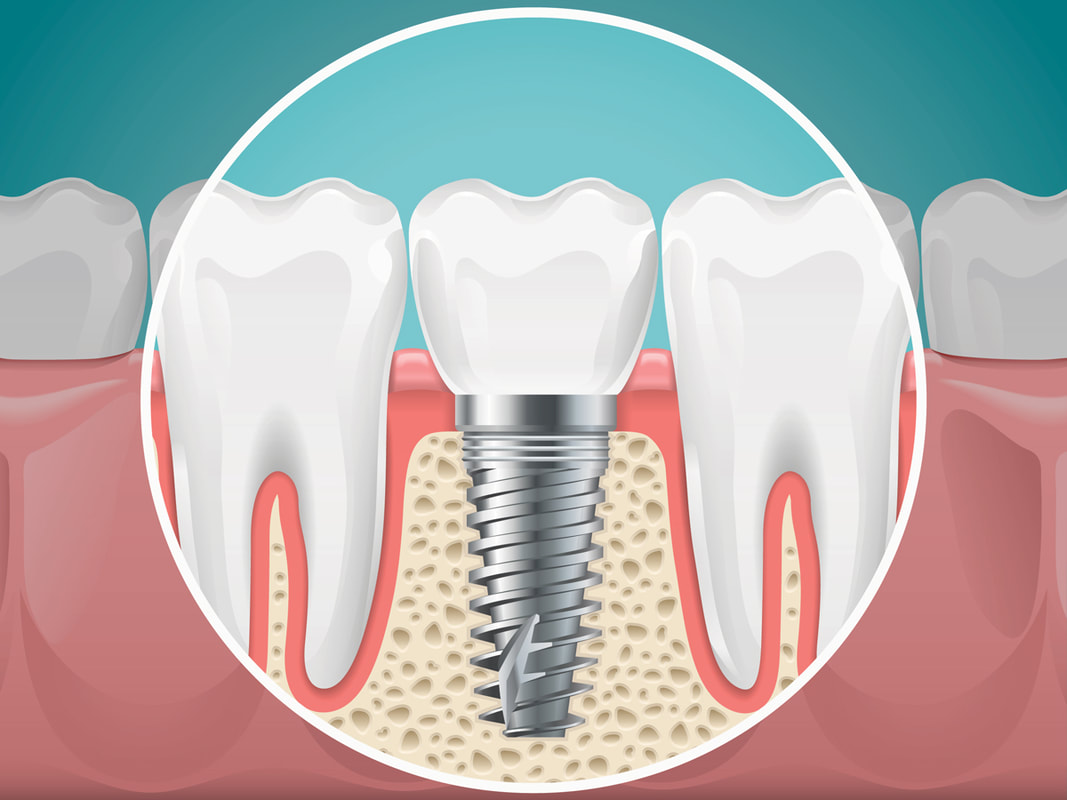
Osseointegration implants are broadly categorized into dental and orthopedic implants. Dental implants are commonly used for tooth replacement, while orthopedic implants are essential for joint replacement surgeries, including hip and knee replacements.
Material Type
Titanium remains the material of choice due to its excellent biocompatibility and strength, making it suitable for both dental and orthopedic applications. Other materials, such as ceramic and zirconia, are also gaining traction, particularly in dental applications where aesthetics play a role.
End-User
The primary end-users include hospitals, dental clinics, and specialized orthopedic clinics, each benefitting from the integration of osseointegration implants into their treatment protocols.
Industry Key Highlights
Growth in the Aging Population: As the global population ages, demand for dental and orthopedic solutions increases, creating a strong market for osseointegration implants.
Rise in Dental Implants: The demand for dental implants is steadily growing as more patients opt for these permanent solutions over temporary fixes.
Technological Innovation: Advances in 3D printing and bioactive materials are enhancing implant quality and safety, driving market growth.
Future Outlook for the Osseointegration Implants Market
Looking ahead, the osseointegration implants market is expected to witness continuous growth, driven by demographic trends, lifestyle changes, and technological advancements. Key factors influencing the future outlook include:
Increased Adoption in Emerging Markets
As healthcare systems improve in emerging markets, the adoption of osseointegration implants is expected to grow, especially in regions such as Asia-Pacific, which is experiencing rapid urbanization and investment in healthcare infrastructure.
Technological Innovations
The integration of advanced technologies, such as patient-specific implants through 3D printing, is expected to gain momentum, making implants safer, more effective, and tailored to individual needs.
Growth in Cosmetic and Functional Applications
Beyond orthopedic applications, there is a growing demand for osseointegration implants in cosmetic surgeries, indicating the potential for expansion in new applications.
Competitive Analysis
The competitive landscape of the osseointegration implants market is marked by a high level of innovation, with companies striving to develop advanced products and technologies. Strategic partnerships, mergers, and acquisitions are common as companies seek to expand their portfolios and enhance their market presence. Collaborations between manufacturers and research institutions are also on the rise, fostering the development of innovative solutions that meet evolving patient needs.
Benefits of the Research Report
Comprehensive analysis of the osseointegration implants market, covering key trends and growth drivers.
Insight into regulatory frameworks and their impact on market growth and product quality.
Detailed competitive analysis and profiles of leading companies.
Segment-wise breakdown for better market understanding.
Future market outlook to guide strategic decision-making.
Frequently Asked Questions (FAQs)
What factors are driving the growth of the osseointegration implants market?
The market is driven by the aging population, increasing prevalence of dental and orthopedic conditions, technological advancements, and a growing preference for minimally invasive procedures.
How does the regulatory scenario impact the osseointegration implants market?
Regulatory frameworks ensure implant quality and safety, fostering trust among healthcare providers and patients. Regions such as Europe and North America have stringent regulations, which uphold high standards in implant manufacturing.
Which companies are leading the osseointegration implants market?
Major players include Medtronic PLC, Stryker Corporation, Smith+Nephew PLC, Zimmer Biomet Holdings, and Institut Straumann AG.
What is the most commonly used material in osseointegration implants?
Titanium is the most widely used material due to its excellent biocompatibility, strength, and corrosion resistance, making it suitable for both dental and orthopedic applications.
What are the future growth prospects of the osseointegration implants market?
The market is expected to grow steadily, driven by emerging trends in minimally invasive surgeries, patient-specific implants, and expanding adoption in emerging markets.
Download Free Sample Report - https://www.techsciresearch.com/sample-report.aspx?cid=26519
Contact
US -
Techsci Research LLC
420 Lexington Avenue, Suite 300,
New York, United States- 10170
Tel: +13322586602
Email: [email protected]
Web: https://www.techsciresearch.com/
Note: IndiBlogHub features both user-submitted and editorial content. We do not verify third-party contributions. Read our Disclaimer and Privacy Policyfor details.