Unlocking the Power of Phenolic Resins: Innovative Applications and Manufacturing Techniques
Strong 8k brings an ultra-HD IPTV experience to your living room and your pocket.
Forget everything you thought you knew about plastic. Phenolic resins, the OG of the polymer world, have been around since the dawn of plastics, and they're still going strong. Invented by Dr. Leo Baekeland in 1907, these versatile materials were the first commercially available plastic, literally shaping the world around us. Remember those iconic black telephones? Yep, those were made of Bakelite, the original brand name for phenolic resins. Buckle up, because we're about to dive into the fascinating world of these durable and adaptable superstars!
Introduction
Phenolic resins represent a highly versatile group of polymers, standing as a testament to the ingenuity of polymer science. Originating at the onset of the polymer age, these resins have continually evolved, expanding their reach into an ever-growing array of applications. Despite their early inception, their development has persisted, leading to their integration into an increasingly diverse range of products and industries.
Here's a breakdown of the two main types of phenolic resins: Novolacs and Resoles
Novolac Resins
Made with a lower ratio of formaldehyde to phenol (less than one)
Cured with an acid catalyst
Typically have a linear molecular structure with methylene and/or ether linkages between the phenol units
Lower molecular weight compared to resoles (around 10-23 phenol units)
Advantages: Excellent chemical resistance, good flame retardancy, highly soluble in various organic solvents
Resole Resins
Made with a higher ratio of formaldehyde to phenol (more than one)
Can be cured with heat or an acid catalyst
Possess a more branched molecular structure due to additional methylene bridges
Higher molecular weight than novolacs
Advantages: Faster curing at lower temperatures, good adhesive properties
Properties of these resins include:
Heat Champions: Stand up to scorching temperatures, making them ideal for fire safety applications.
Material Masters: Used across industries like electronics, construction, and aerospace.
Lightweight Powerhouses: Low density keeps weight down without sacrificing strength.
Cool Customers: Low thermal conductivity keeps things from getting too hot.
Chemical Champs: Resist corrosion and chemicals, making them perfect for harsh environments.
Strength to Spare: High strength-to-weight ratio packs a powerful punch.
Design Delights: Flexible for complex 3D structures, opening design possibilities.
Cost-Effective Champions: Affordable way to create intricate parts.
Built to Last: Excellent fatigue and impact resistance ensure long-lasting performance.
Quiet Achievers: Improve acoustics for a quieter environment.
Under the Radar: Radar and sonar transparency make them valuable for specific applications.
Low Maintenance Marvels: Easy to care for, minimizing upkeep.
Manufacturing Process
Phenolic resins are produced through the reaction between phenols and aldehydes, resulting in the formation of either novolacs or resoles. Subsequently, these resins undergo cross-linking, achieved through either heating in the instance of resoles or the application of curing agents for novolacs. This process results in the creation of chains characterized by a significant presence of aromatic rings, each adorned with a phenol group.
Novolak Resins
Traditionally, Novolac resins have been synthesized utilizing acid catalysts, employing an abundance of phenol. On the other hand, resole and resitole resins are typically prepared using an alkaline catalyst with an excess of formaldehyde, resulting in polymers featuring pendant methylol groups. At the resitole stage, the resins exhibit elevated molecular weights and some degree of cross-linking, leading to heightened viscosity. As each methylol group serves as a potential cross-linking site, resitole resins readily transform into cross-linked, infusible polymers upon heating. However, these resins are inherently unstable.
Conventionally, it's understood that acidic catalysts predominantly yield resins with 4,4'- and 4,2'-linkages, albeit some 2,2'-linkages are also formed. However, acidic catalyzed resins are deemed less suitable when rapid curing is required due to the prevalence of 4,4'- and 4,2'-linkages. In recent times, novolac resins have been developed incorporating substantial proportions of 2,2'-linkages through the utilization of metal oxide or metal salt catalysts. This polymerization process is often termed ionic polymerization. These ortho-resins exhibit accelerated curing and generate cross-linked phenolic resins with enhanced mechanical properties, presumably due to the more organized structure of the polymer molecule achieved with 2,2'-linkages. Nonetheless, the production of such phenolic resins has been restricted to methods employing an excess of phenol to prevent resin gelation during polymerization.
Novolak Process
In the novolak process, the initial step involves charging the reactor with molten phenol, followed by the precise introduction of an acid catalyst. Subsequently, a formaldehyde solution is incrementally added at a temperature of around 90 ºC (194 ºF), with the aim of achieving a formaldehyde-to-phenol molar ratio within the range of 0.75:1 to 0.85:1.
Formaldehyde should be added gradually to maintain safety throughout the process. The reaction proceeds over a duration of 6 to 8 hours at a temperature of 95 ºC. After the reaction period, volatile components, water, and some residual free phenols are removed. The final phenol content in the resin undergoes meticulous monitoring, as it directly influences the resin's properties. This attention to phenol content is crucial, as it dictates the ultimate characteristics of the resin.
Source :Vaisala
Resole Phenolic Resins
Resole Phenolic Resin Formation Process: Resole phenolic resins are synthesized through a process called polymerization. This involves heating a mixture of phenol and formalin (a solution of formaldehyde in water) in the presence of an alkaline catalyst such as ammonia, sodium carbonate, or sodium hydroxide. The reaction typically takes place in a controlled environment, such as a reactor vessel, to ensure optimal conditions for polymerization.
Molar Ratio and Catalyst: The molar ratio of phenol to formaldehyde in the reaction mixture is crucial for controlling the properties of the resulting resin. A ratio of 1:1 or higher is often used. The addition of an alkaline catalyst accelerates the reaction and promotes the formation of the desired resin product.
Polymerization and B-Staging: Polymerization of the phenol and formaldehyde molecules occurs during the heating process. However, it is essential to stop the reaction before reaching the gel point, a stage where the resin becomes too viscous to handle. This intermediate stage is referred to as B-staging.
Intermediate Resole Phenol-Formaldehyde Resin: At the B-stage, the product is an intermediate resin known as resole phenol-formaldehyde resin. This intermediate retains some of the properties of both phenol and formaldehyde while exhibiting thermoplastic behavior.
Drying for Solid Product Formation: If a solid resin product is desired, the intermediate resin is subjected to vacuum drying for several hours. This process removes excess moisture and prevents premature heat hardening of the resin.
Water-Soluble Thermoplastic Nature: Resole phenol-formaldehyde resin is characterized by its water-soluble nature and thermoplastic behavior. It contains methylol groups (CH2OH) that contribute to its solubility in water.
Curing Process: The curing process involves heating the resole resin above its gel point. During curing, the resin undergoes further chemical reactions, forming larger molecules with methylene cross-links. This process transforms the thermoplastic resin into a thermoset material, which is more rigid and durable.
Polycondensation Reaction and By-Product Formation: The resinification reaction between phenol and formaldehyde is a type of polycondensation reaction. In this reaction, water is released as a by-product as the polymer chains grow longer and cross-link with each other.
Short Shelf Life: Resole phenolic resins have a limited shelf life, typically less than a year. This is due to the propensity for the resin to undergo further polymerization or degradation over time, especially in less controlled storage conditions.
One-Step Curing Process: Resole phenolics are often referred to as one-step phenolics because they do not require the addition of external curing agents. The curing process is solely initiated by heating the resin.
Applications: Resole phenolic resins find widespread use in various applications, including casting resins for molding, bonding resins for adhesives, and laminating resins for reinforcing materials such as paper and wood.
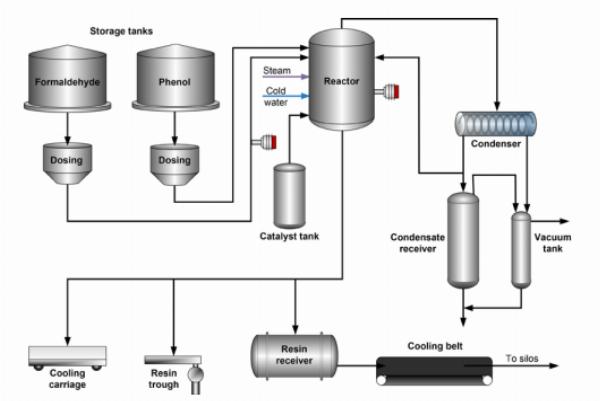
Schematic of typical reactor vessel for the bulk polymerization of phenol-formaldehyde resins
Applications of Phenolic Resins
Phenolic resins, with their impressive heat resistance, chemical stability, and affordability, find applications across various industries. Here are some of their prominent end uses:
Construction:
Wood adhesives: A major application, phenolic resins are the go-to binder for plywood, especially weather-resistant exterior plywood, due to their excellent moisture resistance. Phenolic resins are used to create decorative and functional laminates for countertops and other surfaces. Their heat tolerance makes them ideal binders in brake pads, clutch discs, and other components that experience high friction.
Electrical and Electronics:
Phenolic resins were pioneers in electrical insulation and are still used in circuit boards for their good insulating properties. They act as efficient coatings and adhesives for batteries and copper clad laminates (CCLs) used in consumer electronics.
Molding compounds:
When reinforced with fibers or flakes, phenolics are molded into heat-resistant objects like appliance handles, electrical insulators, and brake linings.
Refractory bricks:
Phenolic resins act as a binding agent in these high-temperature resistant bricks.
Market Outlook:
Phenolic resins are experiencing a surge in popularity due to their remarkable versatility. Across industries like automotive (heat-resistant brake components), construction (durable countertops), and electronics (insulating circuit boards), these resins offer superior performance. Their flame-retardant properties make them ideal for safety-critical applications in aviation interiors and fire doors. Furthermore, ongoing research and development is constantly expanding the range of uses for phenolic resins, solidifying their position as a crucial material for enhancing performance, safety, and durability across a vast array of industries.
Phenolic Resins Major Manufacturers
Major companies in the global Phenolic Resins market are Kolon Industries, Inc., Sumitomo Bakelite Co., Ltd., Hexcel Corporation, Georgia-Pacific Chemicals, DIC Corporation, Kraton Corporation, Hexion, Bostik, Inc., and SI Group, Inc, and Others.
Conclusion:
The future of phenolic resins is bright! Their impressive versatility and adaptability across industries make them a true game-changer. From the searing heat of car brakes to the intricate world of electronics, these resins deliver unparalleled performance. As research continues to unlock new possibilities, phenolic resins are poised to become an even more essential material, shaping a future built on durability, safety, and cutting-edge innovation.
Note: IndiBlogHub features both user-submitted and editorial content. We do not verify third-party contributions. Read our Disclaimer and Privacy Policyfor details.







