Veterinary Reference Laboratory Market Share, Industry Growth Analysis, Size, Report 2025-2033
Strong 8k brings an ultra-HD IPTV experience to your living room and your pocket.
Market Overview:
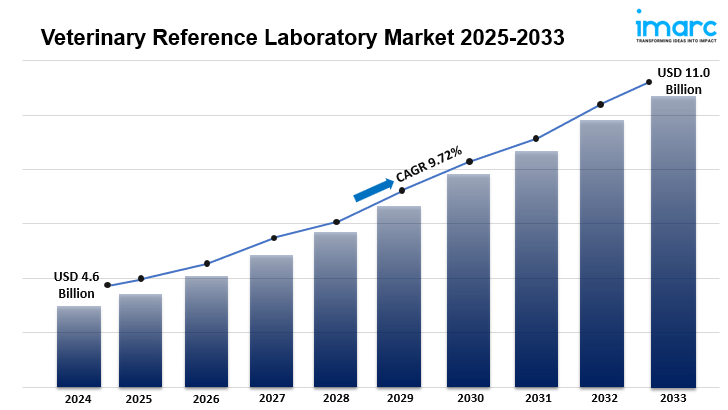
The global veterinary reference laboratory market is experiencing significant growth, driven by the rising incidence of zoonotic diseases, advancements in diagnostic technologies, and increased pet ownership. In 2024, the market reached a value of USD 4.6 billion and is projected to attain USD 11.0 billion by 2033, reflecting a compound annual growth rate (CAGR) of 9.72% during the forecast period.
Study Assumption Years:
• Base Year: 2024
• Historical Year: 2019-2024
• Forecast Year: 2025-2033
Veterinary Reference Laboratory Market Key Takeaways:
• Market Size and Growth: The market was valued at USD 4.6 billion in 2024 and is expected to reach USD 11.0 billion by 2033, with a CAGR of 9.72% from 2025 to 2033.
• Regional Dominance: North America leads the market, attributed to advanced veterinary healthcare infrastructure and high pet ownership rates.
• Technological Advancements: There's a notable shift towards molecular diagnostics and point-of-care testing in veterinary practices.
• Rising Pet Ownership: Increased awareness of animal health and a surge in pet ownership are propelling market demand.
• Regulatory Impact: Stringent regulatory mandates are enhancing the quality and reliability of veterinary diagnostics.
• Market Segmentation: The market is segmented by technology, application, and animal type, catering to diverse diagnostic needs.
Market Growth Factors:
Emergence of New Diagnostic Technologies: The veterinary reference laboratory sector is growing by leaps and bounds in terms of technological improvements. Implementation of molecular diagnostics, including real-time polymerase chain reaction and DNA sequencing, has improved accuracy and speed of animal disease detection. Now, it is entirely possible for a veterinarian to identify a pathogen in an animal at the molecular level, so that early intervention and effective treatment can be achieved. Furthermore, the incorporation of automation and digital technology into laboratory operations improves operational efficiencies and reduces turnaround time of processes. These types of technology are pertinent for fulfilling demands requiring accurate and fast delivery of veterinary diagnosis.
Implementation of Stringent Regulatory Mandates: Stricter guidelines have been established by governments and regulatory authorities throughout the world resulting from the increasing cost of diagnosis in animals. These governmental and regulatory provisions place restrictions on the quality and reliability of veterinary diagnostics; require nationwide standardized testing procedures with validated tools; and, therefore, will promote credibility in the actual test result. This also ensures continuous advancements in laboratory practice and equipment to meet most of the stringent standards, resulting in investments in top-notch options in technology. Certainly, this regulatory environment reassures pet owners and livestock producers of reliable diagnostics and leads the market into consistent growth through establishing a culture of quality and accountability.
Increase in a Prevalence of Zoonoses: The emergence of zoonotic diseases-the disease in which there is direct oder undetected human transmission from the diseased animal to others- strengthens the considerable need for effective veterinary diagnostics. Cases such as avian influenza and rabies demonstrate how animal health can be directly correlated and sometimes intimately linked to public health. They have raised awareness on the need for more intensive testing and surveillance of animal populations, particularly among food-producing animals and companion animals. Comprehensive diagnostic services from a veterinary reference laboratory will prove helpful in early detection and management of such diseases, thus avoiding potential public health risks and improving market growth.
Request Sample For PDF Report: https://www.imarcgroup.com/vascular-stents-market/requestsample
Market Segmentation:
Breakup by Technology:
• Clinical Chemistry
• Hematology
• Immunodiagnostics
o ELISA
o Lateral Flow Rapid Tests
o Others
• Molecular Diagnostics
o PCR
o Microassays
o Others
• Others
Breakup by Application:
• Clinical Pathology
• Toxicology
• Productivity Testing
• Others
Breakup by Animal Type:
• Livestock Animals
• Companion Animals
Market Breakup by Region:
• North America (United States, Canada)
• Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
• Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
• Latin America (Brazil, Mexico, Others)
• Middle East and Africa
Regional Insights:
North America leads the veterinary reference laboratory market, driven by advanced veterinary healthcare infrastructure and high pet ownership rates. The region's focus on animal health and adoption of cutting-edge diagnostic technologies contribute significantly to market dominance.
Recent Developments & News:
The veterinary reference laboratory market is experiencing notable advancements, particularly in molecular diagnostics and point-of-care testing. The integration of digital technologies and automation in laboratory processes is enhancing efficiency and accuracy. Additionally, the expansion of veterinary healthcare facilities and services is broadening access to advanced diagnostics, meeting the growing demand for animal health services.
Key Players:
• Boehringer Ingelheim GmbH
• IDEXX Laboratories Inc.
• Neogen Corporation
• Phoenix Central Laboratory for Veterinarians Inc. (Zoetis Inc.)
• ProtaTek International Inc. (Pharmgate LLC)
• Royal GD
• Texas A&M Veterinary Medical Diagnostic Laboratory
• VCA Inc. (Mars Incorporated)
• Virbac Animal Health India Private Limited
Note: If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Note: IndiBlogHub features both user-submitted and editorial content. We do not verify third-party contributions. Read our Disclaimer and Privacy Policyfor details.