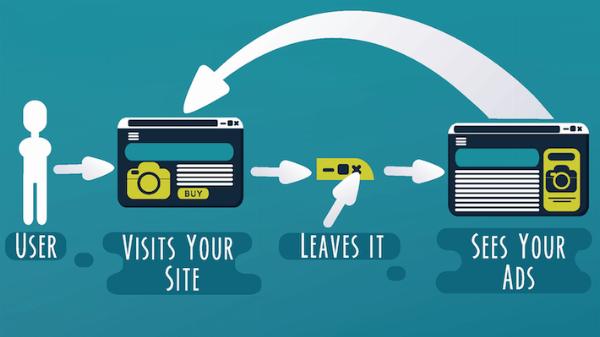
On-Page SEO Optimization – Fix Hidden Errors Killing Rankings!
On-Page SEO Optimization – Fix Hidden Errors Killing Rankings!
What Are the Best Practices for Packaging Medical Devices to Ensure Safety and Compliance?
Written by David » Updated on: June 17th, 2025

Packaging medical devices correctly is crucial for maintaining their sterility, safety, and integrity. Proper packaging not only protects the device but also ensures compliance with regulatory standards. Following best practices in medical device packaging can help manufacturers meet these requirements and safeguard patient health.
Here are some essential practices to follow:
Understand Regulatory Requirements
Regulatory compliance is critical while packaging medical devices. Different countries have specific regulations governing medical device packaging. It is essential to understand and adhere to these standards to avoid legal issues and ensure patient safety. Familiarize yourself with regulations such as ISO 11607, which specifies requirements for packaging systems for sterilized medical devices. Staying updated with any changes in these regulations is also vital for ongoing compliance.
Select Appropriate Packaging Materials
Choosing the right packaging materials is crucial for maintaining the sterility and integrity of medical devices. Materials should be strong enough to protect the device from physical damage and environmental factors like moisture and contaminants. Common materials include medical-grade paper, Tyvek, and plastic films. Ensure that the materials used are compatible with the sterilization process and do not compromise the device's safety or functionality. The packaging should also be easy to open without compromising sterility.
Implement Sterilization-Compatible Design
The packaging design must accommodate the sterilization method used. Whether it is ethylene oxide, gamma radiation, or steam sterilization, the packaging must withstand the process without degrading. Design features such as vents for gas sterilization or durable seals for radiation sterilization should be incorporated. This compatibility ensures that the device remains sterile until it is used. Conduct thorough testing to ensure the packaging performs well under different sterilization conditions.
Perform Rigorous Testing
Testing is a crucial step in ensuring the safety and compliance of medical device packaging. Conduct various tests to evaluate the packaging's strength, integrity, and barrier properties. Tests should simulate real-world conditions like transportation, storage, and handling. Common tests include burst strength tests, seal strength tests, and microbial barrier tests. Rigorous testing helps identify potential weaknesses and ensures the packaging can withstand the rigors of the medical environment.
Labeling and Documentation
Proper labeling and documentation are essential for compliance and safety. Labels should include important information such as the device name, lot number, expiration date, and sterilization method. Clear and accurate labeling helps healthcare providers use the device correctly and safely. Maintain detailed records of the packaging process, including material specifications, test results, and compliance certifications. Documentation is crucial for audits and regulatory inspections, demonstrating adherence to required standards.
Conclusion
Packaging medical devices with safety and compliance in mind is essential for protecting patient health and meeting regulatory requirements. By understanding regulatory requirements, selecting appropriate materials, ensuring sterilization compatibility, performing rigorous testing, and maintaining proper labeling and documentation, manufacturers can achieve safe and compliant packaging. These best practices help ensure that medical devices remain sterile and functional from production to use, ultimately contributing to better patient outcomes and trust in healthcare products.
FAQs
Q: What are the key regulatory standards for medical device packaging?
A: Key regulatory standards include guidelines from the FDA, EMA, and ISO, specifically ISO 11607, which outlines requirements for materials, sterile barrier systems, and packaging processes.
Q: Why is material selection important in medical device packaging?
A: Material selection is crucial because the materials must be durable, sterile, and compatible with the device, ensuring its protection and maintaining sterility throughout its shelf life.
Q: How does packaging design impact medical device safety?
A: Effective packaging design protects the device from damage, contamination, and environmental factors while ensuring easy opening and clear identification, enhancing overall safety.
Q: What role does sterilization compatibility play in packaging?
A: Sterilization compatibility ensures that the packaging can withstand the sterilization process without compromising sterility or functionality, maintaining the device's safety and effectiveness.
Q: How important is sustainability in medical device packaging?
A: Sustainability is increasingly important, with a focus on using eco-friendly materials and practices that minimize environmental impact without compromising safety and compliance.
Note: IndiBlogHub features both user-submitted and editorial content. We do not verify third-party contributions. Read our Disclaimer and Privacy Policyfor details.
Copyright © 2019-2025 IndiBlogHub.com. All rights reserved. Hosted on DigitalOcean for fast, reliable performance.