AAV Contract Development and Manufacturing Organizations Market Growth & Opportunities 2032

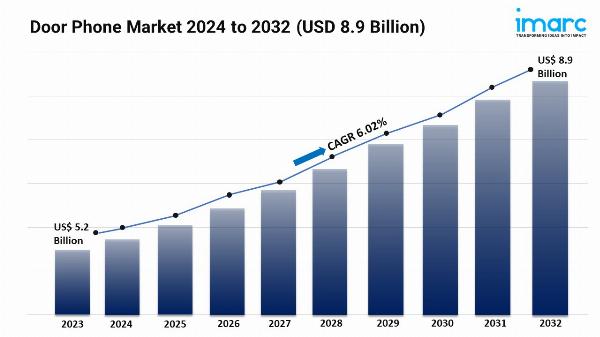
IMARC Group's report titled "AAV Contract Development and Manufacturing Organizations Market Report by Workflow (Upstream Processing, Downstream Processing), Culture Type (Adherent Culture, Suspension Culture), Application (Cell and Gene Therapy Development, Vaccine Development, Biopharmaceutical and Pharmaceutical Discovery, Biomedical Research), End User (Pharmaceutical and Biopharmaceutical Companies, Academic and Research Institutes), and Region 2024-2032", offers a comprehensive analysis of the industry, which comprises insights on the global AAV contract development and manufacturing organizations market growth. The global market size reached US$ 675.1 Million in 2023. Looking forward, IMARC Group expects the market to reach US$ 2,500.3 Million by 2032, exhibiting a growth rate (CAGR) of 15.66% during 2024-2032.
For an in-depth analysis, you can refer sample copy of the report: https://www.imarcgroup.com/aav-contract-development-manufacturing-organizations-market/requestsample
Factors Affecting the Growth of the AAV Contract Development and Manufacturing Organizations Industry:
Advancements in Gene Therapy:
The growing demand for AAV vectors in gene therapy delivery is offering a positive market outlook. Advancements in gene therapy techniques are leading to the development of more sophisticated vector designs and delivery strategies. AAV development and manufacturing organizations (CDMOs) are continually adapting their manufacturing processes to accommodate these advancements and ensure the production of vectors that meet evolving specifications. In addition, AAV CDMOs are leveraging bioprocessing technologies, such as improved cell culture systems, purification methods, and analytics, to streamline production workflows and increase yield while maintaining product quality and consistency.
Outsourcing Trend:
The rising focus on outsourcing AAV vector manufacturing is bolstering the market growth. In addition, biopharmaceutical companies are leveraging the capabilities of AAV CDMOs to mitigate risks, accelerate production timelines, and allocate resources more efficiently. By outsourcing, firms can access advanced manufacturing technologies and ensure compliance with regulatory standards without capital investment. This trend underscores the strategic advantage of partnering with specialized CDMOs, enabling pharmaceutical companies to focus on core competencies while benefiting from the specialized capabilities offered by AAV CDMOs.
Technological Advancements:
Innovations in AAV vector production, purification, and analytics are impelling the market growth. Advanced technologies enhance manufacturing efficiency, scalability, and product quality, meeting the increasing demands of gene therapy developers. Besides this, CDMOs are investing in novel platforms and processes to gain a competitive edge and attract clients seeking advanced manufacturing solutions. Furthermore, modular bioprocessing systems enable AAV CDMOs to customize manufacturing workflows, optimize resource utilization, and expedite process development and scale-up. These platforms facilitate cost-effective manufacturing solutions, empowering AAV CDMOs to meet the evolving demands of gene therapy.
Leading Companies Operating in the Global AAV Contract Development and Manufacturing Organizations Industry:
- ABL Manufacturing (Institut Mérieux)
- Asklepios BioPharmaceutical Inc. (Bayer AG)
- Anlongbio
- Belief Biome Inc.
- Catalent Inc.
- Charles River Laboratories International Inc.
- Creative Biogene
- GenScript ProBio (GenScript Biotech Corporation)
- Merck KGaA
- Oxford Biomedica
- TFBS Bioscience Inc.
- Thermo Fischer Scientific Inc.
AAV Contract Development and Manufacturing Organizations Market Report Segmentation:
By Workflow:
- Upstream Processing
- Downstream Processing
Downstream processing represents the largest segment as it assists in ensuring that the final product meets stringent regulatory standards.
By Culture Type:
- Adherent Culture
- Suspension Culture
Adherent culture holds the biggest market share on account of the rising focus on maintaining cell viability and productivity.
By Application:
- Cell and Gene Therapy Development
- Vaccine Development
- Biopharmaceutical and Pharmaceutical Discovery
- Biomedical Research
Cell and gene therapy development accounts for the largest market share due to the increasing need for personalized and targeted treatments.
By End User:
- Pharmaceutical and Biopharmaceutical Companies
- Academic and Research Institutes
Pharmaceutical and biopharmaceutical companies exhibit a clear dominance in the market, driven by the rising focus on core competencies.
Regional Insights:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America enjoys a leading position in the AAV contract development and manufacturing organizations market, which can be attributed to the presence of biopharmaceutical and biotechnology companies.
Global AAV Contract Development and Manufacturing Organizations Market Trends:
The growing demand for AAV CDMO services due to the expanding pipeline of gene therapy candidates across therapeutic domains like rare diseases and oncology is positively influencing the market. In addition, biopharmaceutical companies are increasingly seeking specialized expertise and scalable manufacturing solutions from CDMOs to expedite product development and commercialization. Besides this, the rising investment in outsourcing partnerships to efficiently navigate the complexities of AAV vector production and purification is bolstering the market growth.
Furthermore, collaborations and partnerships between AAV CDMOs and biopharmaceutical companies assist in accelerating product development and commercialization.
Note: If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us:
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARCs information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the companys expertise.
Our offerings include comprehensive market intelligence in the form of research reports, production cost reports, feasibility studies, and consulting services. Our team, which includes experienced researchers and analysts from various industries, is dedicated to providing high-quality data and insights to our clientele, ranging from small and medium businesses to Fortune 1000 corporations.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145 | United Kingdom: +44-753-713-2163
Note: IndiBlogHub features both user-submitted and editorial content. We do not verify third-party contributions. Read our Disclaimer and Privacy Policyfor details.