Dive Deep into Acrylamide: Unveiling Applications, Producing Techniques, and Revolutionary Technologies
Strong 8k brings an ultra-HD IPTV experience to your living room and your pocket.
Let's chat about a fascinating little compound called Acrylamide. But here's where it gets really interesting: Acrylamide isn't just your average chemical – oh no! It's a monomer, which means it's the building block for all sorts of cool polymers. We're talking about stuff like Polyacrylamide– sounds nice, right?
Now, hold onto your hats because here's the fun part: these polymers are everywhere! From keeping our water squeaky clean in water treatment plants to helping drill for oil and gas, making paper, and even weaving textiles, Acrylamide-based wonders are hard at work in all sorts of industrial and commercial gigs. But wait, there's more! Acrylamide also plays a role in cosmetics, as well as lending a hand in adhesives and grouts.
Introduction
Acrylamide, a versatile building block, acts as a thickener in cosmetics, printing inks, and adhesives. It also helps purify water by attracting impurities and strengthens fabrics. Polyacrylamides, the polymerized form of Acrylamide, take things a step further. In the paper industry, they bind fibers and improve pigment retention. In construction, they add strength to cement and regulate water loss. They also find uses in paints, coatings, and durable applications like car parts and appliances. From thickening lotions to strengthening buildings, Acrylamides and polyacrylamides are key materials across many industries. Beyond its major applications, Acrylamide finds uses in various specialized areas. It acts as a thickener for latex products, stabilizes emulsions in printing inks, and gels explosives for controlled detonation. Acrylamide also serves as a binder in adhesives and tapes and plays a role in the production of diazo compounds used in some dyes. Additionally, scientists utilize Acrylamide in specialized techniques like gel chromatography and electrophoresis for separating molecules in research settings.
Manufacturing Process
Synthetic Process
Acrylamide Production Process: The process typically starts with acrylonitrile, often made by reacting propylene with ammonia and oxygen (ammoxidation). Varieties of copper-based catalysts employed in this process are:
(A) a blend of a copper ion and metallic copper in the guise of copper wire or powder,
(B) reduced copper acquired through the reduction of a copper compound via a reducing agent,
(C) decomposed copper derived from the breakdown of a copper compound through heat, among other means, and
(D) Raney copper obtained by treating a Raney copper alloy with an alkali, among other methods. Reduced copper is yielded through these processes.
Preferably, it is advisable to prevent exposure of these copper-based catalysts to oxygen and oxygen-containing gases both prior to and during their utilization. Oxygen has a detrimental effect on their catalytic efficacy and leads to an increase in by-products such as ethylene cyanohydrin.
Typically, acrylonitrile derived from the ammoxidation of propylene serves as the initial acrylonitrile in the hydration reaction outlined in this invention. This acrylonitrile typically contains impurities like acetone, acrolein, oxazole, acetonitrile, propionitrile, methacrylonitrile, as well as cis- and trans-crotonitriles, with hydroquinone monomethyl ether utilized as a stabilizer.
The catalytic hydration reaction uses acrylonitrile in conjunction with the specified copper-based catalyst. The reaction occurs either in a suspended or fixed catalyst bed within the liquid phase, achieved by passing the reactants through the catalyst bed using either a flowing or batchwise approach. The weight ratio of acrylonitrile to water employed in the hydration reaction is largely flexible. Ideally, the preferred weight ratio of acrylonitrile to water ranges from 60:40 to 5:95, particularly falling within the range of 50:50 to 10:90. The reaction temperature for the hydration process is preferably maintained within the range of 70°C to 150°C, with a preference for temperatures between 90°C to 140°C. Acrylonitrile conversion rates are preferably targeted between 10% to 98%, with a particular emphasis on achieving rates ranging from 30% to 95%.
Typically, the pressure in this process ranges from atmospheric pressure to 10 atmospheres. However, since dissolved oxygen present in the catalyst, acrylonitrile, water, and Acrylamide (used as a co-solvent) can diminish the catalyst's activity and elevate the formation of undesirable by-products, it is preferable to completely eliminate oxygen prior to introducing these materials into the reactor.
The resulting reaction solution extracted from the reactor subsequent to the hydration reaction comprises unreacted acrylonitrile, unreacted water and Acrylamide, alongside trace amounts of copper and by-products like ethylene cyanohydrin.
The reaction solution derived from the aforementioned reaction undergoes a conventional evaporation or distillation process to yield a concentrated aqueous solution of Acrylamide while reclaiming nearly all of the unreacted acrylonitrile and a portion of the water. The reclaimed acrylonitrile and water are typically reused as starting materials, although they may find application in other processes.
In the described process for producing Acrylamide through the hydration of acrylonitrile with a copper-based catalyst, acetone, present as an impurity in the initial acrylonitrile, experiences minor loss during the reaction process. Nonetheless, the majority is recuperated through distillation alongside the unreacted nitrile and subsequently recycled back into the reaction system. With continued operation, however, the concentration of acetone within the reaction system gradually rises.
Biological process
Isolation of Nitrile-utilizing microorganisms
Nitrile-consuming microorganisms are isolated from soil samples using an enrichment culture method, employing a medium containing 0.2% (v/v) nitrile, with or without 0.5% (w/v) glycerol in the basal medium. These microorganisms are capable of utilizing various nitriles including acetonitrile, propionitrile, acrylonitrile, isobutyronitrile, succinonitrile, glutaronitrile, adiponitrile, and triacrylonitrile as their sole source of carbon.
Screening and Assay Method for Acrylamide Producing Strains
The isolated strains are cultured aerobically at 28°C for 3 days on the isolation medium. Subsequently, the cells were centrifuged, washed with physiological saline, and suspended in 0.1 M potassium phosphate buffer at pH 7.0. To screen for Acrylamide-producing strains, the reaction mixture consists of 100 µmol of potassium phosphate buffer at pH 7.0, 300 µmol of acrylonitrile as substrate, and wash cells from 3 ml of culture broth, in a total volume of 1.0 ml. The reaction proceeds at 30°C for 1 hour with moderate shaking and stopped by adding HCl. The amount of Acrylamide formed in the reaction mixture is quantified using a Shimadzu gas-liquid chromatograph, equipped with a flame ionization detector. A glass column, packed with Porapack Q are used. Operational parameters are set as follows: column temperature at 210°C; injection and detector temperature at 240°C. Nitrogen is used as the carrier gas.
Preparation of cell-free extract and enzyme assay
The suspended cells obtained as described above are disrupted for 10 minutes on ice with a Kaijo-denki 19kHz ultrasonic oscillator. After disruption, the cells are centrifuged at 17,000 x g for 20 minutes at 5°C. The resulting supernatant solution is dialyzed overnight against 0.01 M potassium phosphate buffer, pH 7.0, containing 1 mM 2-mercaptoethanol.
Isolation of Acrylamide
The reaction mixture, which contains Acrylamide, undergoes centrifugation to separate the cells. Following centrifugation, the supernatant is lyophilized and subsequently treated with methanol for extraction. After centrifuging to remove insoluble residue, the extract is evaporated under vacuum at room temperature. The resulting crude crystals are dissolved in warm methanol and then filtered. Recrystallization from warm methanol yields colorless crystals.\
Major Acrylamide Processes used by Leading Players.
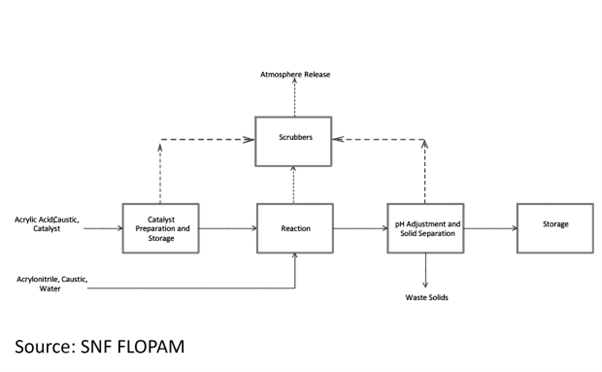
SNF Flopam India Pvt. Ltd. is a subsidiary of SPCM SA France, the parent company of the SNF Floerger group, renowned for its expertise in producing and processing water-soluble polymers. In the process used by SNF FLOPAM, Acrylamide production involves four reaction vessels. Precise control is essential for both the amounts of ingredients (acrylonitrile, water, catalyst, and several sodium compounds) and the reaction environment (temperature and acidity). Acrylonitrile levels are critical to protect the catalyst. After the initial reaction, two additional tanks ensure all the starting material is converted to Acrylamide, keeping unwanted acrylonitrile below 100 ppm. To maintain the lifespan of both the catalyst and the product, they add specific sodium compounds and adjust acidity with sodium hydroxide solution. Safety measures include using nitrogen to smother any fires and strict temperature control to avoid unwanted clumping (polymerization) of the Acrylamide. Reactors have built-in cooling systems to aid in this precise temperature control.
MCI AAM Process is a process used by Mitsui Chemicals for the production of Acrylamide. This method offers several advantages. It utilizes a highly active biological catalyst, which significantly reduces the amount of raw materials and utilities needed. The reaction itself is very efficient, occurring at room temperature and normal pressure. The biocatalyst excels at converting the starting material (acrylonitrile) into the desired product (Acrylamide) with minimal waste. Following the reaction, a special filtration process effectively removes the biocatalyst from the product mixture. The final result is a 50% Acrylamide solution, conveniently obtained without any further concentration steps. This biocatalytic approach presents a highly efficient and environmentally friendly way to produce Acrylamide.
Applications of Acrylamide
Water Treatment
Acrylamide acts like a tiny magnet in wastewater treatment. It attracts and clumps together suspended dirt and tiny organic materials floating in the water. These clumps, called flocs, become heavy and sink to the bottom, leaving clean water behind. This process is essential for removing impurities from both municipal wastewater (from homes and businesses) and industrial wastewater (from factories).
Enhanced oil recovery:
Acrylamide isn't directly used in oil recovery, but its modified version, partially hydrolyzed polyacrylamide (HPAM), is essential in Enhanced Oil Recovery (EOR). HPAM boosts water viscosity, slowing its movement and enabling better oil pocket penetration in reservoirs. This enhances the efficiency of oil recovery.
Paper-making
Acrylamide is used to make Polyacrylamide which is used as a binder and retention aid in papermaking. It helps to bind the paper fibers together and to retain fillers and pigments in the paper.
Flocculating agent
Polyacrylamide, derived from Acrylamide, has the ability to absorb water and cause particles to clump together makes it useful in a variety of thickening and flocculation applications, such as sewage treatment, soil conditioning, and ore processing.
Market Outlook:
The Acrylamide market is driven by its crucial role in water and wastewater treatment across various industries. Since Acrylamide acts as a flocculant, efficiently removing impurities and particles from water, it's essential for municipal, industrial, and commercial sectors. This dominance is further amplified by the growing footprint of oil and gas, chemical, pharmaceutical, and food & beverage industries, all of which rely heavily on clean water processes. Secondly, Acrylamide's role as a building block, particularly for polyacrylamide in wastewater treatment, fuels market demand. Furthermore, the rising use of Acrylamide monomers in diverse sectors like gel electrophoresis, ore processing, and even dye and plastic synthesis strengthens the market.
Acrylamide Major Manufacturers
Significant companies in the Global Acrylamide market are Ashland Global Holding Inc., SNF, Kemira OYJ, BASF SE, EMCO Dyestuff, Mitsui Chemicals Global, Pvt. Ltd., Ineos AG, Solvay, Nuoer Group, Black Rose Industries Ltd., and Others.
Conclusion:
Acrylamide and its polymerized form, polyacrylamide, prove themselves to be surprisingly versatile across a wide range of industries. From thickening our favorite cosmetics to aiding in oil drilling efficiency, these chemicals play a behind-the-scenes role in many aspects of our daily lives. Acrylamide acts as a thickening and stabilizing agent in everything from shampoos and lotions to printing inks and adhesives. Meanwhile, polyacrylamides find use in water treatment, paper production, and construction materials, enhancing everything from wastewater treatment to textile goods.
Note: IndiBlogHub features both user-submitted and editorial content. We do not verify third-party contributions. Read our Disclaimer and Privacy Policyfor details.







