Document Management Software with AI Automation
Strong 8k brings an ultra-HD IPTV experience to your living room and your pocket.
Qualityze offers an advanced Document Management Software with AI automation, designed to streamline the creation, control, storage, and distribution of critical business documents. Built on the robust Salesforce platform, this cloud-based solution enables organizations to manage the complete document lifecycle while ensuring compliance with regulatory requirements such as ISO, FDA 21 CFR Part 11, and GxP. With intelligent automation capabilities, it minimizes manual efforts and enhances efficiency, accuracy, and consistency in document management processes.
At the heart of Qualityze’s solution is its AI-powered automation, which intelligently handles repetitive tasks such as document classification, metadata tagging, version control, and review scheduling. The AI engine can auto-suggest relevant tags or categories based on content, improving searchability and retrieval times. It also supports automated routing of documents for review and approval, reducing bottlenecks and ensuring timely updates. These capabilities not only speed up processes but also reduce the risk of human error.
The software offers a centralized and secure repository with role-based access controls to protect sensitive information and maintain data integrity. Users can easily create, edit, and collaborate on documents in real-time while maintaining an auditable trail of changes. Document templates and controlled workflows further standardize document creation across the organization. AI automation ensures that document expiration dates, reviews, and training updates are automatically monitored and triggered as needed.
Qualityze’s document management system is also fully integrated with other EQMS modules such as Training Management, Change Control, and CAPA. When a document is updated, for example, it can automatically initiate training assignments or change requests, ensuring compliance and organizational alignment. Dashboards and analytics provide insights into document status, user activity, and workflow progress, supporting informed decision-making and continuous improvement.
In summary, Qualityze’s EQMS Suite Document Management Software with AI automation empowers organizations to manage documents more effectively, reduce administrative overhead, and maintain regulatory compliance. The AI-driven features enhance productivity, ensure accuracy, and provide real-time visibility into document activities. Whether managing SOPs, policies, manuals, or technical files, this solution helps organizations achieve greater control, standardization, and operational efficiency in their document processes.
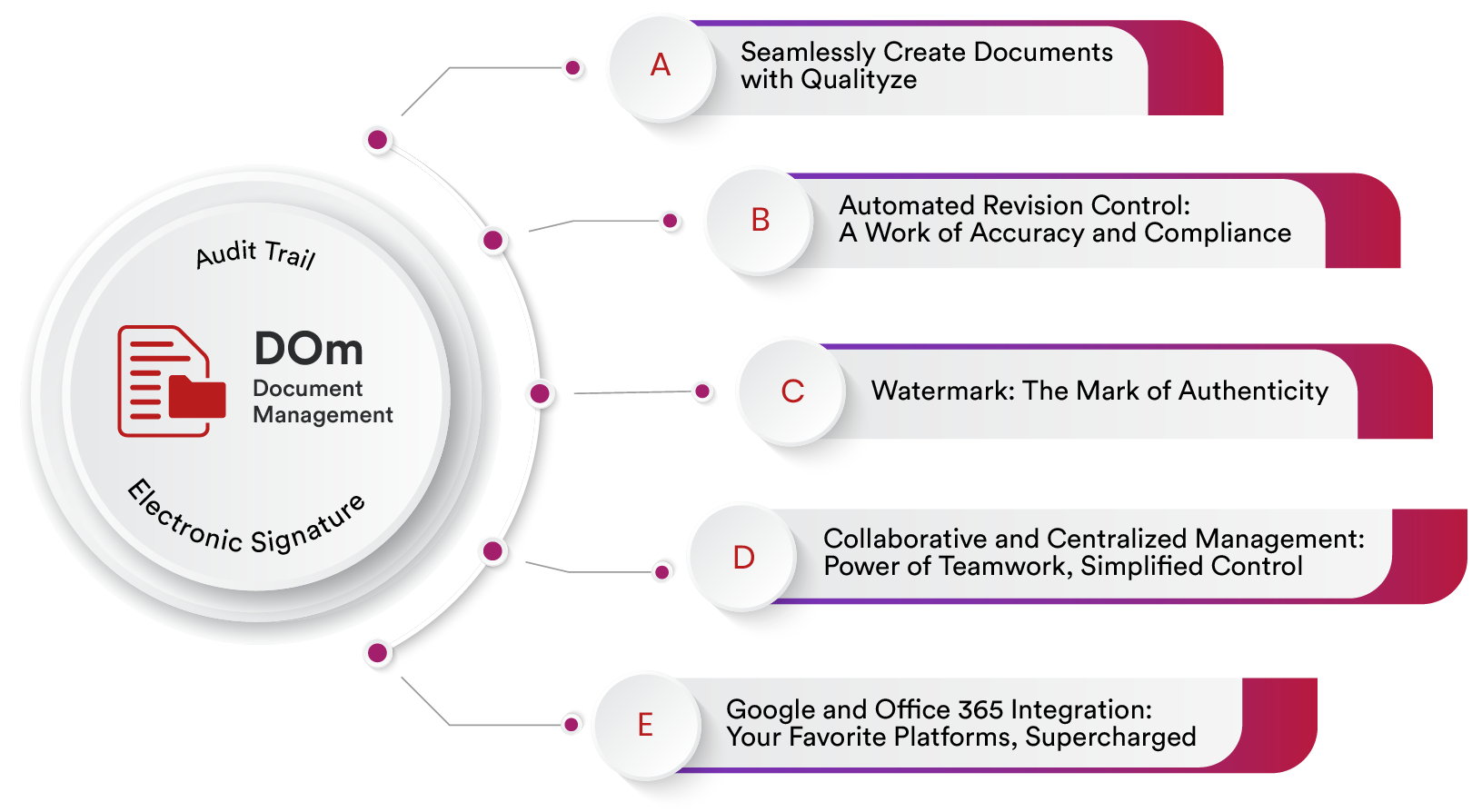
Best Features of Enterprise Document Management Software - Qualityze
Qualityze Document Management Software offers a comprehensive set of features designed to streamline the creation, control, collaboration, and distribution of documents across the organization. Built on the Salesforce platform, the software provides a secure, cloud-based environment where users can create, review, approve, and store documents in a structured and compliant manner. It supports all types of controlled documents, including SOPs, work instructions, policies, and technical files, while ensuring full version control and audit trails.
One of the key features is automated document lifecycle management, which includes configurable workflows for review, approval, release, and obsolescence. The software supports automated reminders and escalations to ensure timely actions and maintain compliance with industry regulations such as ISO, FDA 21 CFR Part 11, GxP, and ICH. With role-based access controls, organizations can ensure that only authorized personnel can create, modify, or approve documents, enhancing data security and integrity.
The platform also excels in collaboration and integration, allowing real-time editing, comment tracking, and electronic signatures. Users can collaborate across departments and locations while maintaining a single source of truth. Integration with other Qualityze EQMS modules such as Training Management, Change Control, and CAPA ensures seamless alignment between documentation and broader quality processes. For instance, an updated SOP can automatically trigger training tasks or initiate a change request.
Additionally, Qualityze EQMS Software provides intelligent search, AI-based classification, and customizable templates that enhance user productivity and document organization. The system supports metadata tagging, document linking, and detailed reporting for complete visibility into document status and performance metrics. With dashboards and analytics, quality managers can easily monitor review cycles, pending approvals, and compliance trends, making it easier to enforce standardization and continuous improvement throughout the organization.
Qualityze Enterprise Document Management Software is a comprehensive, cloud-based solution designed to help large organizations efficiently manage their critical documents throughout the entire lifecycle. Built on the robust and secure Salesforce platform, this software supports organizations in maintaining compliance with global regulatory standards such as ISO, FDA 21 CFR Part 11, GxP, and ICH, making it an ideal choice for regulated industries like life sciences, manufacturing, healthcare, and aerospace. Its scalable architecture and advanced features cater to the complex needs of enterprise environments where document control, collaboration, and compliance are critical.
One of the key strengths of Qualityze’s enterprise document management solution is its end-to-end document lifecycle management capabilities. The software automates key processes such as document creation, review, approval, distribution, and archiving through configurable workflows. These workflows ensure that documents follow a standardized process with role-based access controls, which guarantees that only authorized personnel can perform actions on documents. Automated alerts, reminders, and escalations help organizations stay on track with review cycles and deadlines, minimizing the risk of outdated or uncontrolled documents.
Key Features
Collaboration is another essential feature in the Qualityze platform. Users across multiple departments and global locations can work on documents simultaneously, with real-time editing, version control, and audit trails that provide full visibility of changes made. The system supports electronic signatures to authenticate approvals and maintain a legally defensible record of document acceptance. Integration with other Qualityze EQMS modules such as Change Management, Training Management, and CAPA ensures that document updates automatically trigger related quality actions, such as initiating training assignments or revising standard operating procedures, fostering seamless alignment across enterprise quality processes.
Additionally, Qualityze leverages AI-powered automation and intelligent search capabilities to enhance productivity. The software can automatically classify documents, tag metadata, and suggest relevant categories, making it easier to locate information quickly and accurately. Customizable templates, comprehensive reporting, and visual dashboards provide quality managers and executives with insights into document status, compliance metrics, and bottlenecks, supporting data-driven decision-making. With secure cloud hosting and multi-level access controls, Qualityze Enterprise Document Management Software offers the flexibility, control, and visibility large organizations need to standardize document management, improve regulatory compliance, and support continuous improvement initiatives across the enterprise.
Note: IndiBlogHub features both user-submitted and editorial content. We do not verify third-party contributions. Read our Disclaimer and Privacy Policyfor details.







