Bulk Content Creation – Scale Without Sacrificing Quality!
Bulk Content Creation – Scale Without Sacrificing Quality!
Left Atrial Appendage Closure Devices Market Size, Share, Growth, and Forecast 2024-2032
Written by stanley huds » Updated on: June 17th, 2025

Global Left Atrial Appendage Closure Devices Market Statistics: USD 6.3 Billion Value by 2032
Summary:
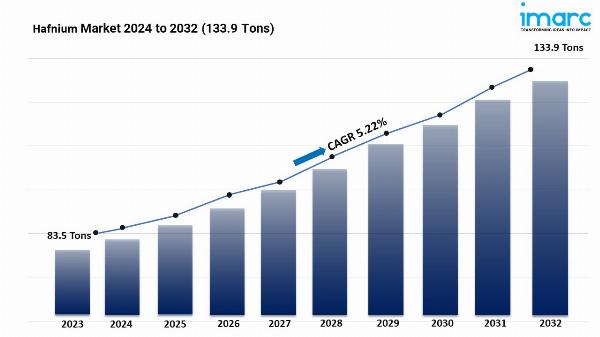
- The global left atrial appendage closure devices market size reached USD 1.5 Billion in 2023.
- The market is expected to reach USD 6.3 Billion by 2032, exhibiting a growth rate (CAGR) of 16.85% during 2024-2032.
- North America leads the market, accounting for the largest left atrial appendage closure devices market share.
- Endocardial LAA devices account for the majority of the market share in the product segment due to the increasing availability of advanced imaging and delivery systems.
- Hospitals hold the largest share in the left atrial appendage closure devices industry.
- The rising incidence of atrial fibrillation (AFib) is a primary driver of the left atrial appendage closure devices market.
- Technological advancements and limitations of oral anticoagulants are reshaping the left atrial appendage closure devices market.
Industry Trends and Drivers:
- Rising prevalence of atrial fibrillation (AFib):
AFib is associated with a higher risk of stroke because it can lead to the formation of blood clots in the left atrial appendage. These clots can then travel to the brain, causing a stroke. As the incidence of AFib rises, especially in the aging population, the need for stroke prevention solutions becomes more urgent. LAA closure devices offer a non-pharmacological method to reduce the risk of stroke in these patients. AFib is more common among the elderly, and with the global population aging, the number of AFib cases is rising sharply. As a result, there is growing demand for treatments that address stroke risk in AFib patients, including LAA closure devices. The World Health Organization (WHO) projects that by 2050, the population over the age of 60 will have doubled, significantly increasing the potential patient pool for these devices.
- Limitations of oral anticoagulants:
One of the major drawbacks of oral anticoagulants, such as warfarin and direct oral anticoagulants (DOACs), is the increased risk of bleeding, particularly gastrointestinal and intracranial bleeding. Long-term anticoagulant therapy poses significant bleeding risks, especially in elderly or frail patients. Many AFib patients, particularly those at high risk of bleeding, cannot safely continue anticoagulants. LAA closure devices provide a safer, non-pharmacological alternative to manage stroke risk without long-term exposure to these bleeding risks. Anticoagulants require strict adherence to dosage and regular monitoring, especially in the case of warfarin, which requires routine blood tests to monitor clotting levels (INR). Many patients struggle with maintaining this regimen, either due to side effects or the inconvenience of monitoring. Non-adherence can result in inadequate stroke protection or increased bleeding risks.
- Technological advancements:
Innovations in LAA closure devices are leading to enhanced designs that offer better sealing of the left atrial appendage, reducing the risk of stroke more effectively. Modern devices are smaller, more flexible, and designed to minimize complications during and after implantation. These improvements have made the procedure safer and more efficient, encouraging wider adoption among cardiologists and patients. Advances in catheter-based technologies have made LAA closure procedures minimally invasive, reducing the need for open-heart surgery. With these technological developments, the procedure can now be performed through a percutaneous approach, requiring only small incisions. This results in shorter hospital stays, faster recovery times, and fewer complications, making LAA closure an attractive option for a broader range of patients.
Request for a sample copy of this report: https://www.imarcgroup.com/left-atrial-appendage-closure-devices-market/requestsample
Left Atrial Appendage Closure Devices Market Report Segmentation:
Breakup By Product:
- Endocardial LAA Devices
- Epicardial LAA Devices
Endocardial LAA Devices represent the largest segment because they are minimally invasive, offer higher safety and efficacy, and are widely preferred for percutaneous procedures over surgical options.
Breakup By End User:

- Hospitals
- Ambulatory Surgery Centers
- Others
Hospitals hold the biggest market share as they are the primary centers for performing complex cardiac procedures like LAA closure, with access to advanced technologies and specialized medical staff.
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America enjoys the leading position in the left atrial appendage closure devices market due to its advanced healthcare infrastructure, high prevalence of atrial fibrillation, and favorable reimbursement policies.
Top Left Atrial Appendage Closure Devices Market Leaders:
The left atrial appendage closure devices market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies. Some of the key players in the market are:

- Abbott Laboratories
- AtriCure Inc.
- Boston Scientific Corporation
- Cardia Inc.
- Lepu Medical Technology (Beijing) Co. Ltd.
- LifeTech Scientific
Note: If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145
Note: IndiBlogHub features both user-submitted and editorial content. We do not verify third-party contributions. Read our Disclaimer and Privacy Policyfor details.
Copyright © 2019-2025 IndiBlogHub.com. All rights reserved. Hosted on DigitalOcean for fast, reliable performance.