Precision in Action: How Knock-in Cell Lines Advance Molecular and Drug Discovery Research
The field of gene editing has rapidly advanced from blunt genetic disruptions to precision-controlled genomic modifications. Among the most powerful applications of this evolution is the creation of knock-in (KI) cell lines, which allow researchers to insert custom DNA elements—such as reporter genes, tags, or regulatory sequences—into a precise location within the genome.
These genetically modified cell lines are invaluable tools in modern biology. They offer direct insight into gene function, enable real-time observation of cellular processes, and form the backbone of many high-throughput drug screening platforms.
This article introduces the key concepts behind knock-in cell line construction, discusses the most common strategies, and explores their broad applications in biomedical research.
What Is a Knock-in Cell Line?
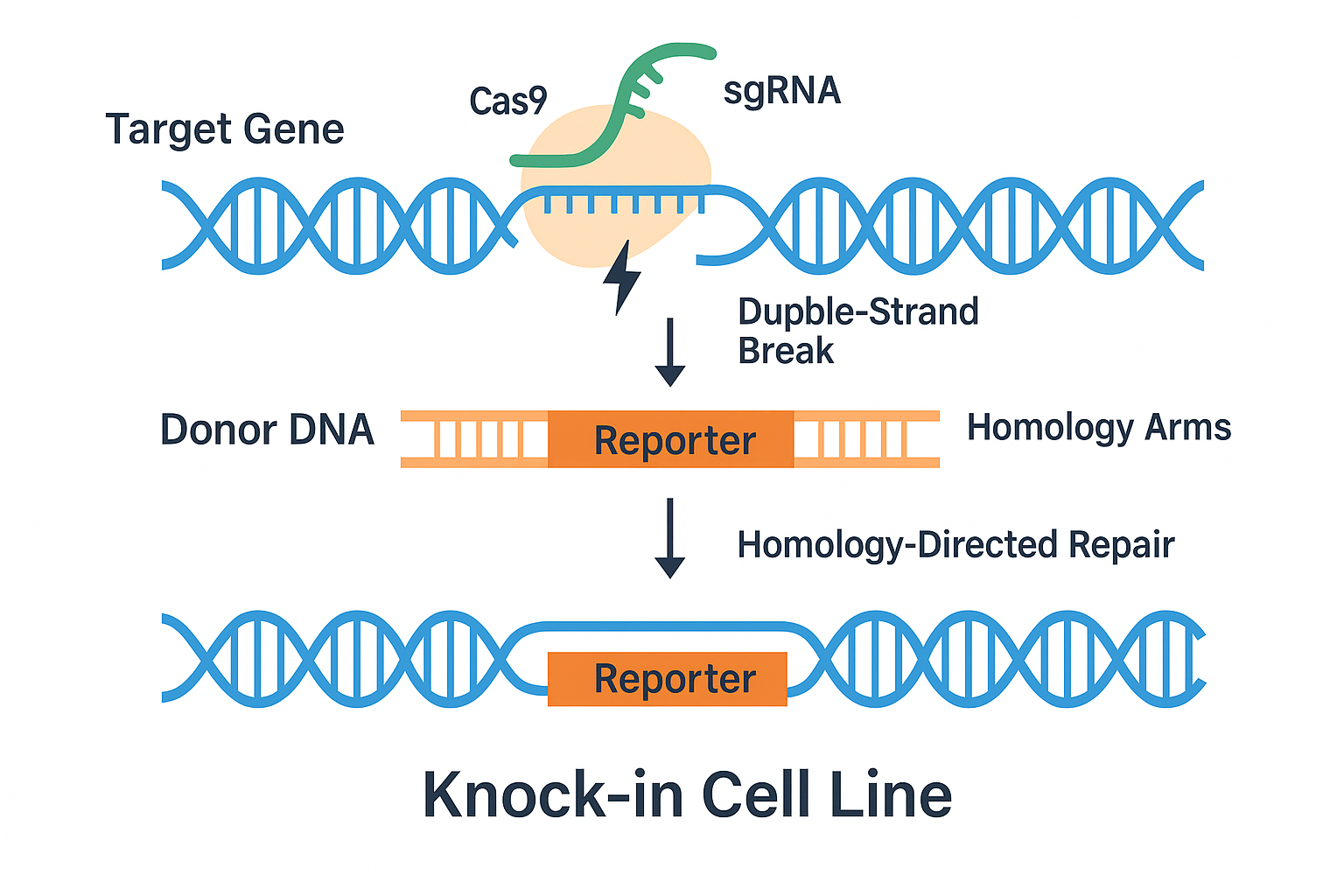
A knock-in cell line is a genetically engineered cell in which a specific DNA sequence has been inserted at a defined genomic locus using homology-directed repair (HDR) mechanisms. This approach enables precise integration of exogenous elements, such as fluorescent proteins (e.g., GFP), luminescent reporters (e.g., luciferase), or epitope tags (e.g., FLAG), into endogenous genes.
Unlike traditional overexpression systems—which often rely on artificial promoters and random genomic integration—knock-in strategies ensure that the inserted sequence is regulated by the gene's natural promoter and chromatin context, preserving physiological relevance.
Gene Editing Tools Behind Knock-in Engineering
Modern knock-in technology is primarily powered by CRISPR-Cas9, a programmable endonuclease system adapted from bacterial immune defenses. Cas9 is directed to a specific genomic site by a guide RNA (gRNA), where it introduces a double-strand break. When a DNA repair template with homology arms is supplied, the cell may use HDR to incorporate the desired sequence during the repair process.
There are two main pathways cells use to repair Cas9-induced breaks:
Non-Homologous End Joining (NHEJ): Quick but error-prone, often resulting in insertions or deletions—commonly used in gene knockouts.
Homology-Directed Repair (HDR): Enables accurate insertion of new genetic material, making it essential for knock-in cell line generation.
While HDR is less efficient than NHEJ, various strategies—including small molecule enhancers, optimized donor templates, and synchronized cell cycles—can increase success rates.
Common Knock-in Strategies: Where and How to Insert the Reporter?
Depending on the research goal, different knock-in designs can be used. Here are four popular approaches:
1. Promoter-Proximal Insertion
The reporter is inserted near the gene’s transcription start site or within the first intron, preserving upstream regulatory elements. This approach captures real-time transcriptional activity.
2. Fusion Protein Tagging
A reporter gene is fused in-frame to the N- or C-terminus of the target gene. This enables visualization of protein localization and dynamics without overexpression artifacts.
3. Co-expression Systems (IRES/2A)
Internal ribosome entry site (IRES) or 2A peptide sequences allow simultaneous expression of the native protein and a separate reporter, maintaining functional integrity of the protein of interest.
4. Complete Replacement
The entire coding region of a gene is replaced with a reporter, often to functionally disrupt the gene while still tracking its promoter activity.
Each of these strategies has trade-offs in terms of expression fidelity, protein function, and ease of validation. Choice depends on whether the aim is to monitor transcription, track protein movement, or measure downstream pathway activity.
Functional Applications of Knock-in Cell Lines
1. Monitoring Endogenous Gene Activity
Knock-in reporters enable researchers to visualize gene expression patterns in real time and under native regulation. This is crucial when studying transcriptional dynamics in response to stimuli, such as drug treatments or environmental changes.
Example: In a model studying lipid metabolism, a luciferase gene was knocked into the SREBP1 locus. This allowed scientists to correlate luciferase signal with promoter activity during metabolic shifts—facilitating the screening of regulatory compounds.
2. Protein Localization and Dynamics
Fusing fluorescent tags like GFP to endogenous proteins allows tracking of subcellular trafficking, interaction dynamics, and turnover rates without the need for antibodies or overexpression.
Example: Knocking in GFP at the MAP1LC3B locus enables the monitoring of autophagy. Researchers observed how autophagosome formation changed in response to rapamycin or chloroquine, using the fluorescence signal as a proxy.
3. Drug Screening and Target Validation
Reporter knock-in cell lines offer high signal-to-noise ratios and reproducibility, making them ideal for high-throughput assays.
Example: A multi-tagged HeLa line—with fluorescent labels for nuclear, cytoskeletal, and autophagy components—was used to evaluate kinase inhibitors. Compounds that induced autophagic vesicle accumulation were identified through fluorescence imaging, confirming their pharmacological impact.
Advantages Over Traditional Methods
Compared to episomal or viral expression systems, knock-in models provide:
Endogenous expression levels, avoiding overexpression artifacts
Stable integration, minimizing variability between replicates
Quantitative output, especially when using luminescent reporters
Dynamic imaging, with high resolution in live-cell conditions
Scalability, supporting high-throughput automation in drug discovery
Outlook: Knock-in Cell Lines in Future Biomedical Research
As single-cell analysis, live-cell imaging, and AI-powered screening continue to evolve, reporter knock-in models will become increasingly central to functional genomics and translational medicine. Emerging technologies such as base editors and prime editors may further refine knock-in precision, while new delivery systems will improve access to hard-to-transfect cell types and organoids.
In the long term, knock-in engineering will not only accelerate drug discovery and diagnostics but also support synthetic biology and cell-based therapy development—where precise, traceable gene expression is vital.
Note: IndiBlogHub features both user-submitted and editorial content. We do not verify third-party contributions. Read our Disclaimer and Privacy Policyfor details.