A Practical Guide to Culturing and Gene Editing of GL261 Glioma Cells
The GL261 cell line, derived from a chemically induced glioma in C57BL/6 mice, has become a cornerstone model in glioblastoma (GBM) research. Its unique ability to grow in immunocompetent hosts sets it apart from human glioma models, allowing exploration of tumor-immune interactions in syngeneic mouse systems.
In this article, we’ll dive into GL261’s biological characteristics, culture practices, common troubleshooting, and gene editing considerations—equipping researchers with practical knowledge to support reliable and reproducible glioma studies.
1. Why GL261 Matters in Glioma Research
GL261 cells offer a valuable balance between biological relevance and experimental accessibility.
- Immunocompetence: GL261 cells can be implanted into C57BL/6 mice, preserving an intact immune system. This is critical for studying immunotherapies such as PD-1/PD-L1 blockade, CAR-T cells, and NK-based therapies.
- High genetic stability: Compared to some patient-derived glioma models, GL261 is relatively stable and well-characterized at the genomic level.
- Versatility: Frequently used in radiation, chemotherapy, and combinatorial immunotherapy research.
Studies have shown that GL261 tumors mimic key features of human GBM, including invasiveness and partial responsiveness to immunomodulatory agents (Szatmári et al., J Neurooncol. 2006).
2. Morphology and Growth Profile
- Cell Type: Epithelial-like, adherent morphology
- Doubling Time: ~24–30 hours under standard culture conditions
- Culture Medium: DMEM supplemented with 10% fetal bovine serum (FBS)
- Gas and Temperature Conditions: 5% CO₂, 95% air, 37°C
- Passage Frequency: Every 2–3 days, ideally at 80–90% confluence
- Recommended Passage Ratio: 1:2 to 1:3
GL261 cells adhere strongly and form dense monolayers. During healthy growth, cells exhibit clear borders with minimal debris. Over-confluency or poor-quality serum may lead to fragmented morphology or increased secretion.
3. GL261 Thawing and Initial Seeding
When recovering cryopreserved GL261 cells from cryovials:
- Rapidly thaw the vial in a 37°C water bath (under 1 minute)
- Transfer contents into a tube with prewarmed complete medium
- Centrifuge at 1100 rpm for 4 minutes to remove DMSO
- Resuspend cells in fresh medium and seed into a T25 flask (or equivalent dish)
- Incubate at 37°C, 5% CO₂, and monitor attachment and morphology after 24 hours
✅ Tip: Avoid seeding cells at very low densities immediately after thawing; moderate density helps improve post-thaw survival.
4. Passaging Protocol and Best Practices
GL261 cells should be passaged at ~80% confluence to avoid nutrient depletion and poor reattachment post-trypsinization, which can occur if cells are allowed to reach full monolayer formation.
Key Steps:
- Rinse cells with PBS to remove serum (which inhibits trypsin)
- Add 1–3 mL of 0.25% trypsin (depending on flask size) and incubate for 1–2 min
- Once cells round up, neutralize trypsin with complete medium
- Gently pipette to dislodge cells and avoid excessive bubbling
- Transfer to a 50 mL tube, centrifuge, and reseed at recommended density
✅ Tip: Trypsin exposure time should be minimal—prolonged digestion increases the risk of cell death or altered gene expression.
5. Cryopreservation and Recovery
GL261 cells freeze well when properly handled. To ensure high post-thaw viability:
- Freeze cells at a density of 5–10 × 10⁶ cells/mL in medium containing 10% DMSO
- Use a controlled-rate freezing container or stepwise -80°C protocol
- Transfer to liquid nitrogen for long-term storage
During recovery, seed cells into smaller culture surfaces (e.g., 6-well plates) before expanding to larger flasks.
6. Managing Common Culture Problems
| Problem | Likely Cause | Suggested Solution |
| Heavy cell debris or low viability | Overgrowth, bad serum, mechanical stress | Use fresh FBS, adjust passage ratio, reduce pipetting force |
| xcess secretion | Overconfluence, metabolic stress | Wash cells with PBS every 2–3 passages |
| Clumped cells | Incomplete digestion, high seeding density | Optimize trypsin time and reduce passage density |
| Poor post-thaw recovery | Low cryo density or fast thaw | Freeze at high density; thaw quickly and gently |
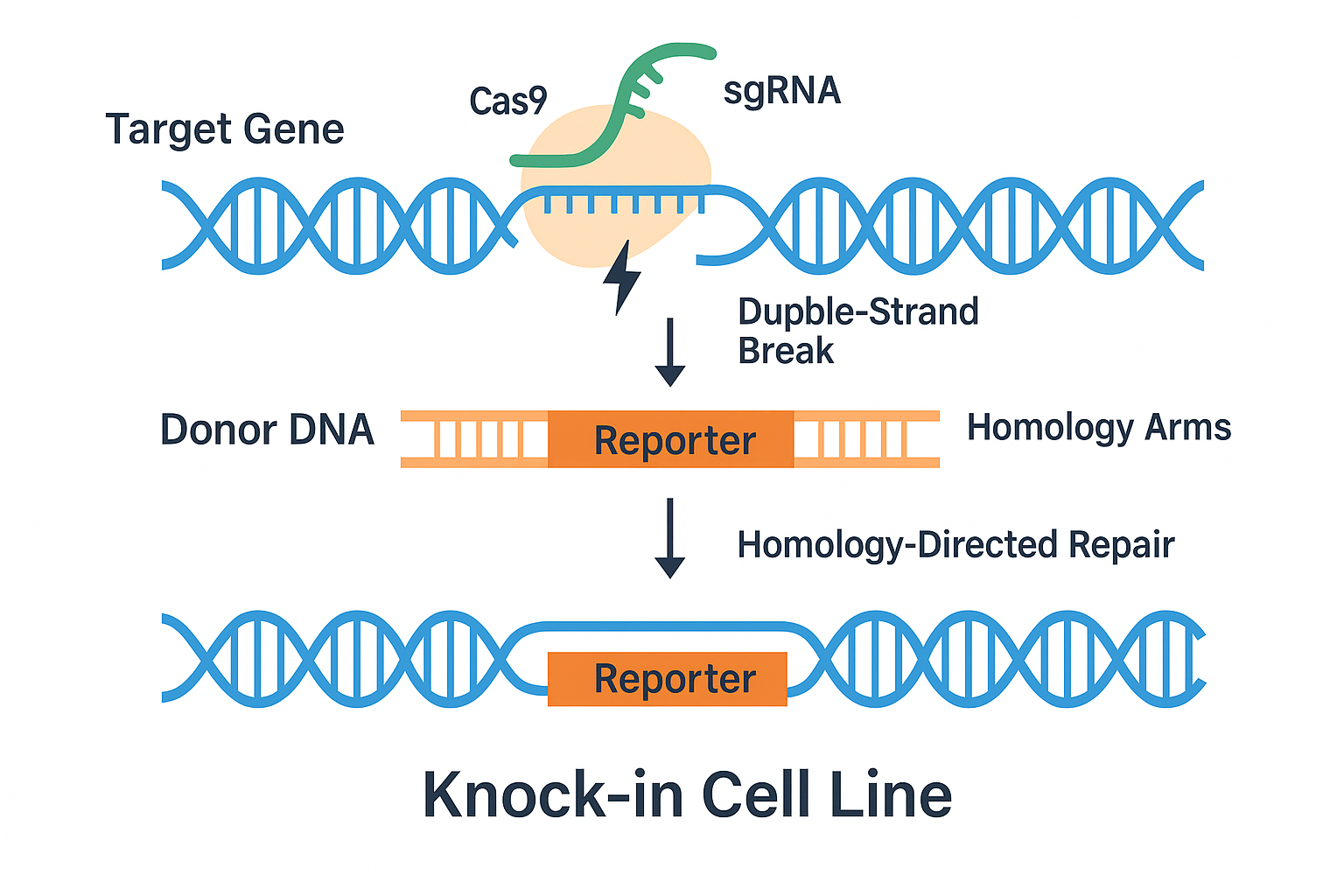
7. Gene Editing in GL261 Cells
Owing to their adherent nature and compatibility with murine models, GL261 cells are ideal for CRISPR/Cas9-mediated gene editing and functional genomic screens.
Editing Options:
- Plasmid transfection (e.g., lipofection): Lower efficiency but useful for transient expression
- Electroporation of RNP complexes: Higher precision and reduced off-target effects
- Lentiviral delivery: Best for stable integration, ideal for creating knockout or reporter lines
Optimization tips:
- Ensure viability >90% before editing
- For electroporation, test voltage/capacitance combinations to reduce cell death
- For viral transduction:
- Pre-titrate MOI (multiplicity of infection)
- Use Polybrene (typically 4–8 μg/mL) to enhance efficiency
- Apply selective antibiotics only after confirming infection success
8. Clonal Expansion and Monoclonal Line Development
To isolate edited clones after gene editing:
- Start with high-viability cells (>90%)
- Perform limiting dilution or single-cell FACS sorting
- Use conditioned medium or supplement-rich plates to support early colony formation
- Wash cells thoroughly to reduce extracellular secretions that may interfere with attachment
Final Notes
GL261 cells remain an indispensable model for glioma biology, particularly in immunocompetent systems. Their utility spans tumor immunology, gene editing, drug screening, and cell therapy development. With proper handling, GL261 cultures are stable and reproducible, making them ideal for translational research settings.
Whether you're dissecting immune checkpoint pathways, testing novel therapies, or building CRISPR-edited syngeneic models, mastering GL261 cell line techniques is essential for generating impactful, reliable data.
Note: IndiBlogHub features both user-submitted and editorial content. We do not verify third-party contributions. Read our Disclaimer and Privacy Policyfor details.