 Guaranteed SEO Boost: Triple Your Rankings with Backlinks starting at 5$
Guaranteed SEO Boost: Triple Your Rankings with Backlinks starting at 5$
Nano-Carrier based Smart Drug Delivery Systems: Taking Aim at Cancer Therapy
Written by divyaochre » Updated on: October 21st, 2024
The creation of smart nanocarrier-based drug delivery systems, also known as smart drug delivery systems (SDDSs), was prompted by the nonspecific dispersion and unpredictable release of pharmaceuticals in conventional drug delivery systems (CDDSs). To lessen the adverse consequences associated with CDDSs, SDDSs can deliver medications to target locations with a lower dose frequency and in a spatially controlled way. Chemotherapy is commonly used to treat cancer, the world's second most common cause of death. The SDDSs have sparked a lot of attention as a potential alternative to chemotherapy because of their site-specific drug delivery. SDDSs are interested in smart nanocarriers, which are nanoparticles that deliver drugs. Smart nanocarriers, targeting mechanisms, and stimulation strategies make up a smart drug delivery system.This review highlights the recent development of SDDSs for several smart nanocarriers, including liposomes, micelles, dendrimers, meso-porous silica nanoparticles, gold nanoparticles, carbon nanotubes, quantum dots, and hydrogels. Also, the challenges and future research scope in the field of SDDSs are also presented.
1. Introduction
Cancer is one of the leading causes of death but is surpassed by cardiovascular diseases. Chemotherapy plays a vital role in treating undetectable cancer micro-focuses and free cancer cells. Chemotherapy utilises chemical substances to kill or stop cancer cell development, regarding that cancer cells are developed often much faster than those are healthy ones, fast-developing cells are the main targeting of chemotherapy, but healthy cells are also rapidly growing, chemotherapy medications target those fast-growing healthy cells. By 2030, approximately 13.1 million cancer-related deaths have been predicted by the World Health Organisation (WHO). These situations are usually treated with traditional therapy, but this traditional approach has unwanted side effects on healthy body parts. Conventional drugs, due to their low specificity, cause serious toxicity in our bodies. These pharmaceuticals give low aqueous solubility and are less bioavailability and have less therapeutic benefit.Nanotechnology growth has a major influence on the treatment of cancer.
The selection of a suitable nanocarrier type follows the choice of the appropriate strategies to classify cancer cells. To identify cancer areas, SDDS uses the physiochemical differences between cancer and healthy cells. There are two main approaches to accurately identify the site of the cancer cell. To assess the cancer site indirectly, passive targeting uses an Enhanced Permeability (EPR) effect. Active targeting uses over-expressed cell surface receptor in cancer cells directly as a guided missile to kill cancer cells. The next step is to release drugs at a certain concentration at a certain location. Depending on the nature and smartness of the nanocarriers, drugs can be released from the nanocarriers by external or internal stimuli.The idea of these innovative therapies is either to block signals that help malignant cells grow and divide uncontrollably, to kill cancer cells by inducing apoptosis, to stimulate the immune system, or to target the delivery of chemotherapy agents specifically to cancer cells, to minimise the death of normal cells and to avoid undesirable side effects.
Conventional chemo-therapeutic substances are affected, both normal and tumor cells, that are distributed in a non-specific way across the body. Tissue selectiveness is an important question, given the possibility of advanced pharmacological agents. The dose within the solidtumor is consequently reduced, resulting in inadequate therapy owing to excessive toxicity. The ultimate objective regarding cancer therapy was to improve their patient’s survival duration and quality life by decreasing their systemic toxicity of chemotherapy.
The size of nanocarriers (10–400 nm) was suitable as a medication carrier because they had the benefits to have been able to carry big amounts of drugs, providing extended flow time (when surface PEGylated particularly) and selective facilitating tumor growth through the improved Effect Permeability and Retention (EPR). Nanocarriers could also be useful in the resolution of other conventional drug limitations, involving weak aqueous solubility, poor bioavailability, and undesirable drug pharmacokinetic characteristics. Additionally, transport through nanocarriers was revealed to resolve MDR (multidrug resistance) induced by drug efflux transporters like P-glycoprotein, which is often over-expressed in cells of cancer.
2. Smart Nanocarriers
Particles with at least one dimension on the order of 1 nm to 100 nm are popularly known as nanoparticles. Nanocarriers are nanoparticles that are utilised as transport modules for other drugs. Under external or internal stimulation, conventional nanocarriers are unable to transport and release drugs at the desired concentration at the targeted spot. As a result, traditional nanocarriers are not smart. To make them smart, they must be changed or functionalised. The following properties should be present in smart nanocarriers:
i. Smart nanocarriers should bypass the body's immune system's cleaning process,
ii. they should only accumulate at the intended location; and
iii. smart nanocarriers should release the cargo at the correct concentration at the targeted site under external or internal stimulation.
Eight promising nanocarriers (Fig. 1) are discussed in detail below:
2.1. Liposomes
Liposomes are spherical vesicles consisting of one or more bilayers of phospholipids that can be generated from cholesterol and natural or synthetic phospholipid. Lipophilic medicines embedded in lipid bilayers and hydrophilic materials in the interior aqueous compartment (Senapatiet al., 2018).
Stability, inappropriate drug loading, rapid drug release, and shorter blood circulation durations are only a few of the issues of conventional liposomes. To actively target the cancer site, monoclonal antibodies, antibody fragments, proteins, peptides, vitamins, carbohydrates, and glycoproteins are commonly grafted on the liposome(Noble et al., 2014). Liposomes have various advantages, including active group protection, cell-like membrane structure, minimal immunogenicity, biocompatibility, safety, efficacy, and increased half-life (Li et al., 2019). Based on nanotechnology, applications of liposome are the first pharmaceutical drug products that are approved for cancer and other therapeutic application. pH change, enzyme transformation, redox reaction, light, ultrasound, and microwaves are all examples of external and internal stimulations that smart liposomes respond to.
2.2. Micelles
Micelles has amphiphilic molecules that are arranged from a core of hydrophobic and hydrophilic corona. The interaction of hydrophobic/hydrophilic molecules control the structure of the micelles. The polar parts of the copolymer are attracted to the solvent when it is hydrophilic and its concentration beyond the critical micelle concentration (CMC), while the hydrophobic parts are directed away. In this way, the hydrophobic parts form a core, whereas the hydrophilic parts form a core. This type of arrangement is known as a direct or regular polymeric micelle. When amphiphilic molecules are exposed to a hydrophobic solvent, they produce a reverse micelle, which is a reverse structure (Shin et al., 2016).
Several advantages of micelles are like prolonged self-life, drug character not affected on drug delivery, etc. An appropriate size of micelles to allow the extravasation at the tumor site. Keet al., designed micelles loaded with both thioridazine —which has been reported to kill cancer stem cells— and doxorubicin, providing a promising strategy for breast-cancer treatment by targeting both cancer and cancer stem cells with this combination therapy(keet al., 2014).
2.3. Dendrimers
Dendrimers are monodispersing structure, homogeneous, nano-sized, and highly branched and well-defined symmetric molecules. The size ranges of dendrimers are 2–10 nm in diameter(Anithaet al., 2018).Dendrimers are polymers with various branches that may be represented graphically as a suction ball(Palmerston Mendes et al., 2017). For cancer treatment, paclitaxel, doxorubicin, methotrexate, and cisplatin are loaded into dendrimer nano systems.
Another advantage lies in the fact that bioactive agents may be encapsulated either into the interior of the dendrimers or can be chemically attached or physically adsorbed onto the dendrimer surface(Sanchez-Morenoet al., 2018).Peptides, proteins, carbohydrates, aptamers, antibodies, and other substances can be applied to the surface of dendritic structures to actively target the cancer site. The surface of a dendrimer can also be modified to react to various stimuli, such as light, heat, pH change, protein, and enzyme transformation(Wang H et al., 2016).
2.4. Meso-porous silica nanoparticles
Meso-porous materials contain pores with diameters between 2 to 50 nm (Brühwiler 2010). MSNs contain silica nanoparticles with such a honeycomb-like porous structure (SiO2). Because of its variable particle size (50 nm to 300 nm), uniform and tunable pore size (2-6 nm), high surface area, high pore volume, and biocompatibility, MSNs are being widely investigated (Slowing 2008). (1) ordered meso-porous silica NPs (MCM-41, MCM-48, and SBA-15) and (2) hollow or rattle-type meso-porous silica NPs (Hergt&Andrä 2007). MSNs have recently been developed as nanocarriers for photodynamic therapy (PDT), photothermal therapy (PTT), or both. PTT and PDT, two important types of phototherapies, sparked a lot of interest in various cancer treatments (Liu et al.,2018). MSNs have been studied and found to be promising carriers for biomedical imaging and drug delivery due to their good biocompatibility, high pore volume, uniform pore size distribution, large surface area and further chemical modification on the surface of MSNs to modulate the nanoparticle surface characteristics (Chan et al.,2015).
2.5. Gold nanoparticles
Because of their unique properties, such as adjustable size, huge surface to volume ratio, cheap synthesis, noble optical properties, thermal ablation of cancer cells, and facile surface functionalisation, metallic nanocarriers are of great interest(Salih 2013). Gold nanoparticles (GNPs) trapped the attention of researchers due to their surface plasmon resonance, tuneable and optical characteristics for their use as a drug carrier. They could be made in a variety of forms of 1–150 nm sizes, making their dispersion simpler (Singh et al.,2018). The study has been often to used bleomycin as an anti-cancer medicine. Bleomycin used in research is among the most active natural antitumor medications for chemotherapy clinics in clinical treatments. However, the therapeutic efficacy is restricted owing to the drug’s side effects, most notably lung toxicity. Bleomycin connects to a ‘DNA’ andtrigger the dual helix to unwind and produces radical oxygen reactive species that cause ‘DNA’ strand splits. The sulphate finish of bleomycin connects to the GNPs surface and provides an effective product used in the combination experiment throughout this simple conjugation (Yang et al., 2018).
2.6. Carbon nanotubes
Carbon nanotubes (CNTs) are a kind of fullerene, a group of carbon allotropes that includes a variety of shapes such as hollow spheres, ellipsoids, tubes, and other shapes. Single-walled carbon nanotubes (SWCNT) and multi-walled carbon nanotubes (MWCNT) are the two varieties of CNTs (MWCNT). The CNT's significant optical absorption in the near-infrared zone makes it an excellent choice for photo thermal ablation; also, nanoparticles with diameters ranging from 50 to 100 nm are rapidly consumed. MWCNTs can pass through numerous cellular compartment barriers, but PEGylated SWCNTs can localise in a single cellular compartment.
2.7. Quantum dots
Quantum dots (QDs) are semi conductive nanoparticles (NPs), constructed of periodic II-VI or III-V group in periodic.QDs vary in diameter from 2 to 10 nm, which is equivalent to or below the dimensions of the excitedradius of Bohr. While the medicine is being delivered at the specific site, this nanocarrier might be utilised to see the tumour. A core, a shell, and a capping substance make up the majority of commercially available QDs. A semiconductor material, such as CdSe, is used in the core. A different semiconductor, such ZnS, is employed to create a shell around the semiconductor core. The two-layer QDs with different materials are encased in a cap. For a variety of reasons, QD-based SDDSs have piqued people's curiosity. To begin with, QDs have a very tiny core diameter of 2-10 nm. Just because of that, it can be used as a tracer in other drug delivery methods. Second, QD surface changes can be implemented in a variety of ways because to diverse surface chemistry. Third, QDs' photophysical features provide them an edge in real-time drug-carrying and drug-release monitoring.
2.8. Hydrogels
Hydrogels are hydrophilic polymer networks with variable structures. They can be classified into those that have a linear structure, and those exhibiting polymeric network with three-dimensional covalent crosslinking. Covalent bonds between chains affect the overall properties of the hydrogel (i.e., swelling ability, elasticity, and drug loading capacity). They can absorb from 10-20% (an arbitrary lower limit) up to thousands of times their dry weight in water, a property attributed to the presence of hydrophilic groups in their structure. This makes them very useful as potential drug carriers in the form of micro- or nanoparticles.
Several synthetic hydrogels have been studied for anticancer purposes.For example, Cheng et al. prepared thermosensitive hydrogels based on poly(γ-ethyl-l-glutamate)-poly(ethylene glycol)-poly(γ-ethyl-l-glutamate) triblock copolymers (PELG-PEG-PELG) for localised and sustained delivery of paclitaxel (PTX) in vivo. The results demonstrated that the PTX-incorporated hydrogels could efficiently suppress the tumorproliferation and did not result in obvious side effect.
Fig.1. Schematic diagram showing various types of smart nano-carriers
3. Cancer cell targeting strategies
If the anti-cancer drug-carrying smart nanocarrier survives the body's immune system's cleaning process, the smart nanocarrier locates the malignant spot.The tumor targeted at nanocarriers leads to better pharmacokinetic and pharmacodynamic profiles, controlled and permanent drug release, better specificity, increased internalisation, and intracellular delivery and, above all, to lower systemic toxicity(Huda et al., 2020).There are two forms of targeting in a smart drug delivery system: passive targeting and active targeting (as shown in Fig. 2). The EPR effect is used in passive targeting to find cancer spots. The ligand-receptor method is used in active targeting to pinpoint the final target—thecancer cell. (Hossenet al.,2018).
3.1. Passive targeting
Due to the leaky endothelium of the tumour vasculature, the accumulation rate of drug-loaded nanocarriers in a tumour is substantially greater than in normal tissue. This phenomenon is known as the enhanced permeability effect. The lymphatic system is the body's drainage system. The nanoparticles are retained in the tumor due to a lymphatic system deficit. The increased retention effect is the name given to this type of retention.
The EPR effect refers to both phenomena. The concentration of anti-cancer medications in the tumor might be boosted several times when compared to healthy tissue using this EPR effect.If nanocarriers can prevent immune monitoring and circulate for a long time, the EPR effect will be ideal.At least three features of nanocarriers are of special importance for this reason:
(i) The maximum length of the nanocarriers should be 10–100 nm. In addition, for successful extravasation from fenestration in leaky vasculature, nanocarriers should be significantly less than 400 nm. On the other hand, nanocarriers must be more than 10 nm to prevent kidney filtration; and to prevent a specific capture of the liver, nanocarriers must be less than 100 nm
(ii) Neutral or anionic particle charge for successful renal function removal should be used
(iii) The reticuloendothelial system must conceal nanocarriers that destroy all foreign material by opsonisation followed by phagocytosis.
3.2. Active targeting
As it is with guided missiles, active targeting entails directing drug-carrying nanocarriers to cancer cells. In terms of cell surface receptor and antigen expression, cancer cells and normal cells can be distinguished. Trans-membrane communication is facilitated by cell surface receptors, which are embedded proteins in the cell membrane. Various cell surface receptors (also known as cell markers), such as folic acid and cell surface antigen, are amplified or overexpressed in cancer cells. Targeting ligands are conjugated to drug-loaded nanocarriers. These ligands find their corresponding target on the cancer cell surface, which is overexpressed. Folate, transferrin, antibodies, peptides, and aptamers are just a few of the ligands that have been studied.
Multiple anti-cancer therapies are divided into groups based on the drugs delivery strategy and are known as “ligand target therapy”. The precise distribution of medicines to cancer cells is the fundamental concept of all these therapeutics. A specific receptor is attacked by antibodies (monoclonal antibodies or fragments), interferes withsignal transduction pathways, controls proto-oncogenes in the proliferation and development of cancer cells for example, trastuzumab (anti-ERBB2, Herceptin®), bevacizumab (anti-VEGF, Avastin®) or etarizumab, a humanised anti-αvβ3 antibody (Abegrin). In this case, the active molecule plays the role of ligand targeting and drug targeting. The role of the ligand targeting in combination with therapeutic molecules can be played by antibodies (or fragments).
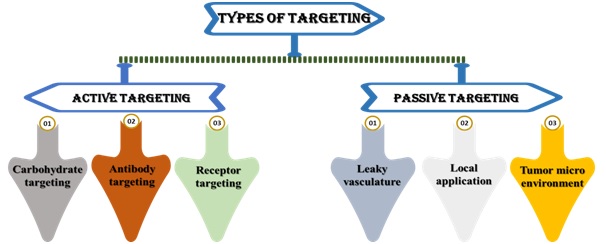
Fig. 2. Types of targeting
4. Stimulus for drug release
Recently, researchers reported and received positive attention NDDS with stimulus-responsive behaviour. In the design of stimulating DDS, two methods may be introduced.The two sorts of stimuli are exogenous and endogenous. Exogenous stimulation is an extra-corporal signal used to release drugs from nanocarriers, such as a magnetic field, ultrasonic waves, an electric field, or a temperature change. An endogenous stimulus is a signal created from inside the body that causes anti-cancer medications to be released. Endogenous stimuli include pH changes, enzyme transformations, temperature changes, and redox reactions.
4.1. Endogenous stimulus
Endogenous stimulus is also known as intrinsic stimulus. In the case of endogenous stimulus, the triggering signal comes from the internal pH level, enzyme activity, redox activity, and temperature changes of the body. Different types of endogenous stimuli are discussed below in detail.
4.1.1. pH responsive stimulus
The Warburg effect states that tumour cells create energy primarily through increased glycolysis, followed by lactic acid fermentation in the cytosol. Cancer cells have a lower pH because of the increased acid production. Because the pH level fluctuates from organ to organ, and even from tissue to tissue, the pH-responsive drug delivery method is intriguing. In tumors, the extracellular pH is acidic, contrasted to the slightly basic internal pH. As a result, several studies have proven pH as an efficient physiological feature for smart medication delivery to tumour locations. Anti-cancer drugs are stored and stabilised in pH-sensitive nanocarriers under physiological pH, but they are promptly released at a pH trigger point, ensuring that intracellular drug concentration reaches a peak.Various techniques, including the insertion of ionizable chemical groups such as amines, phosphoric acid, and carboxyl groups, among others, can be used to achieve the goal. Physical and chemical changes in these groups are pH-dependent, resulting in drug release.
4.1.2. Enzyme stimulus
Enzyme-stimulus nanocarriers are nanocarriers whose surfaces have been changed to make them sensitive to the bio-catalytic action of enzymes. Enzymes are enzymes that catalyse biological processes in living organisms. Enzymes are significant targets for medication delivery because they play a critical role in cell function control. The overexpressed enzyme of the extracellular environment of tumour locations is used in enzyme-triggered methods. Because the intracellular enzyme concentrations of cancer cells and healthy cells are almost identical, this technique is ineffective for intracellular drug release. Proteases, which break down proteins and peptides, are an excellent choice for releasing drugs from liposomes
4.1.3. Redox sensitive stimulus
The highly effective antioxidant is glutathione-sulfhydryl (GSH). There are three amino acids. GSH is present in all mammalian tissue at higher concentrations. The reduction of the microenvironment is controlled by GSH. GSH is at least 4 times higher in a tumor site than normal cells. GSH is 1000 times higher than in the plasma intracellular concentration. GSH, an R-S-S-S functional group, can decrease disulfide bonds of the nanocarriers. Disulfides bonds decreasing leads to the release of encapsulated drug; the disulfide bond of cross-related micelles, for instance, may be reduced at the GHS cell site. Reduced disulfide bonds contribute to the exact unloading of nano-vehicle freight.
4.1.4. Temperature-responsive stimulus
The temperature in the tumor is increased (40–42 ºC) at high rates of aerobic glycolysis and rapid proliferation. The programmed temperature, based on high-rate aerobic glycolytic tumors and quick proliferation, increased temperatures, can thus be calculated by sensitive nanocarriers. Through the difference in temperature between the tumor and standard tissues, programmed nanocarriers can be built. These nanocarriers can alter their structure by releasing antitumor drugs in response to a rise in the temperature of the tumor.
4.2. Exogenous stimulus
Exogenous physical effects are primarily temperature, light, magnetic field, and ultrasound. When these signals act on nanocarriers which respond to exogenous stimuli, drug releases can quickly be caused.
4.2.1. Magnetic field responsive stimulus
An extracorporeal magnetic field is employed in magnetically induced systems to collect drug-loaded nanocarriers in tumor locations following nanocarrier injection. Magnetic stimuli can be achieved using core-shell structured nanoparticles covered with silica, polymer, or magneto liposomes (maghemite nanocrystals encased in liposomes).
4.2.2. Thermo-responsive stimulus
Drug-loaded nanocarriers release their payloads in response to temperature changes in this manner. Nanocarriers change their shape, solubility, or hydrophilic and hydrophobic balance at a specific temperature. When the temperature of a nanocarrier changes, some of them release their payload
4.2.3. Light-triggered stimulus
The development of light-triggered drug delivery in recent years has opened up new possibilities for on-demand drug administration. The wavelengths of the light might be ultraviolet, visible, or near infrared. The stimulus is accomplished by making the nanocarriers light sensitive. CNTs and GNPs are excellent light-triggered stimulus materials, particularly in the near-infrared (NIR) region. To destroy cancer cells, metallic nanocarriers absorb light and convert it to heat.
4.2.4. Ultrasound-responsive stimulus
Because of its non-invasiveness, deep penetration into the body, and non-ionizing irradiation, ultrasound is being studied extensively for releasing pharmaceuticals from nanocarriers. Both thermal and mechanical impacts can be created in nanocarriers utilising ultrasound to release the loaded medication
4.2.5. Electric-field responsive stimulus
The payloads are released via an electric field in this stimulation mechanism. To release medications, the thermo-responsive, light-triggered, and ultrasound-responsive stimulus systems detailed thus far need big or specialised equipment. Electric fields, on the other hand, are simple to create and manipulate. For an electric-responsive stimulus, conducting polymers such as polypyrole (PPy) are being considered. Conducting polymers are used to change nanocarriers, and their effectiveness is determined by the dopant utilised and the drug's molecular weight. Biotin is a dopant that has been investigated in the lab
5. Applications of nanocarriers in treatment of different types of cancer
Nanocarriers like liposome are particularly attractive delivery of drug system which they could be generated and easily modified such that they can be utilised in the therapy of a wide range of cancer. Liposome nanocarriers chemotherapeutics based are used in the breast cancer therapy that liposome is therapeutic index increases even it is encapsulated drugs of anticancer, various drugs like doxil that is liposomal formulation currently used in the breast cancer therapy.
Nanocarriers also used in treatment of liver cancer. In the study, create a simple formulation mixing cytochrome C and treatment of chemotherapy. The research had shown that integrated supply with Cytochrome C (HNP-c) and Paclitaxel (PTX) of active HNP surfaces has generated more significant impacts in cell lines of hepatocellular carcinoma (HepG2), hepatocellular carcinoma cell lines (Huh–7D12) and human endothelial cell cancer (SK-hep1). PTX belongs to the family of taxane anti-microtubular drugs that had shown a powerful impact in the therapy of cancer by the targeting microtubule subunit of β-tubulin. Here the PTX parallel to the microtubule that connects the tubulin and improves the procedures of polymerisation.
A pH-responsive liposome system, containing the glioma tumor cells was studied by Zhao et al. Due to the acidic pH of gliomas, a tumor-sensitive peptide and liposomes were present in the system that could react and release the encapsulated drug to acidic environments. Doxorubicin has been used as a treatment against cancer. In vivo as well as in vitro studies show that the pharmacologically active portion was released once the system reached acidic pH. Consequently, the mechanism provided triggers for acidic pH sensitivity, which proved a suitable candidate for treatment against cancer.
6. Challenges and future perspective
The development of next generation nanocarrier drugs are one of the key challenges faced by the recent advancement in nanotechnology for the successful treatment of different tumors. This expansion will support the complex targeting of tumors through interaction with the surface ligand and the receptors on the cells and tissues selected. However, some challenges need to be addressed, for instance lack of experience, difficulty crossing the cell membrane, a small medicine therapy window, regulatory challenges, and cost-efficiency. New nanotechnology-based tools and therapeutic strategies for the diagnosis and treatment of precautionary cancer will be developed and applied through the development of cancer biology and polymer chemistry. Nanotechnology also contributes to the delivery of medicines for eye treatment and to the production of tissue engineering scaffolds. Nanocarriers are carrying and releasing anti-cancer medicines on the targeted sites to kill the cancer cell. The ultimate destiny of nanocarriers with drugs is concerned. In different vital organs, such as lungs, spleen, kidneys, liver, and heart, typical nanocarriers accumulate according to chemical composition, scale, shape, defined surface area, surface load and lack of shell around the nanocarrier.
7. Conclusion
In conclusion, Nanocarriers, a sensation of current science, play vital roles in biomedical applications, especially in anticancer drug delivery. Significant development in science, major research efforts were conducted to develop DDS which could react to physiochemical changes which are internally or externally in an advanced way with checking and reliability at high level. Cancer is also a highly complex and serious illness with various problems. The nanotechnology has a rapidly evolving research field with ability for scanning, tracking, detect and transmitting drugs to target cells of tumor. Nanocarriers are innovative tumor approaches targeting increased effectiveness and decreased toxicity. Basically, by controlling the surface characteristics and molecular size that provide drugs with less often dosage and better precise and problematic-to-access tissue penetration over a longer period. In the above paper, summarised the importance of the different types of nanocarriers (organic nanocarriers and inorganic nanocarriers) and its ability for applications as site-specific delivery of drug.
Visit now: https://www.pharmafocusasia.com/articles/nano-carrier-based-smart-drug-delivery-systems
Disclaimer:
We do not claim ownership of any content, links or images featured on this post unless explicitly stated. If you believe any content or images infringes on your copyright, please contact us immediately for removal ([email protected]). Please note that content published under our account may be sponsored or contributed by guest authors. We assume no responsibility for the accuracy or originality of such content. We hold no responsibilty of content and images published as ours is a publishers platform. Mail us for any query and we will remove that content/image immediately.
Copyright © 2024 IndiBlogHub.com. Hosted on Digital Ocean

